The regulatory landscape for research peptides is complex, evolving, and varies significantly across jurisdictions. For researchers, healthcare providers, suppliers, and individuals interested in peptides, understanding applicable regulations is essential for ensuring compliance, avoiding legal pitfalls, and conducting work within appropriate legal frameworks.
This comprehensive guide explores peptide regulations at federal and international levels, clarifying legal status, oversight mechanisms, compliance requirements, and best practices for navigating this intricate regulatory environment.
Understanding the Regulatory Framework
Peptide regulation depends on several factors including intended use (research, therapeutic, cosmetic, etc.), chemical structure and properties, route of administration, and jurisdiction (federal, state, international).
Regulatory Categories
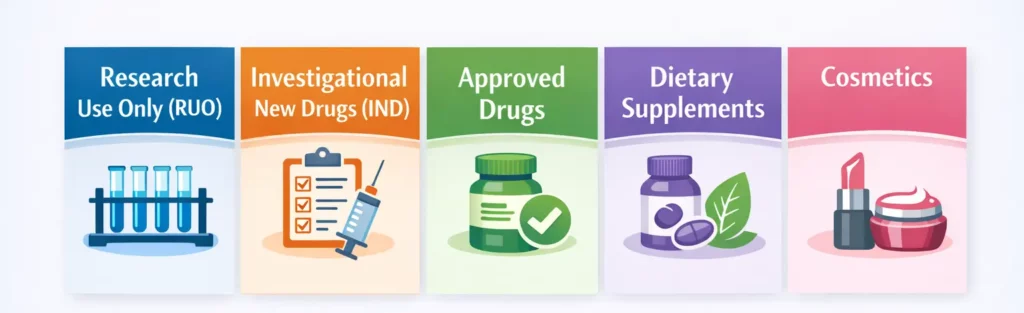
Peptides fall into different regulatory categories based on their intended application:
Research Use Only (RUO)
Peptides labeled “For Research Use Only” are intended for laboratory research, not for human or animal therapeutic use. These peptides undergo minimal regulatory oversight compared to therapeutic products but must comply with research chemical regulations, laboratory safety standards, institutional oversight (IACUC for animal research, IRB for human research), and proper handling and disposal protocols.
Investigational New Drugs (IND)
Peptides being developed as therapeutic agents and tested in humans require IND status from the FDA. This involves preclinical safety data, manufacturing information, clinical trial protocols, informed consent procedures, and ongoing reporting requirements.
Approved Drugs
FDA-approved peptide drugs are regulated as prescription medications. These products must meet pharmaceutical manufacturing standards (GMP), follow approved labeling and indications, and comply with post-market surveillance and reporting requirements.
Dietary Supplements
Some peptides or amino acid sequences may be marketed as dietary supplements under DSHEA. However, this is controversial, as the FDA generally does not recognize peptides that mimic hormones or exert drug-like effects as legitimate dietary supplements.
Cosmetics
Peptides used in skincare products fall under cosmetic regulations, which are less stringent than drug regulations but still require safety assessments, accurate labeling, and adherence to manufacturing standards.
FDA Oversight of Peptides
In the United States, the Food and Drug Administration (FDA) exercises primary regulatory authority over peptides.
FDA Regulatory Authority
The FDA regulates peptides under several statutory authorities, including the Federal Food, Drug, and Cosmetic Act (FD&C Act), the Public Health Service Act, and dietary supplement regulations where applicable.
Drug Classification
Peptides intended to diagnose, cure, mitigate, treat, or prevent disease are classified as drugs requiring FDA approval. Even peptides labeled as “research use only” may be deemed misbranded if marketed with implied therapeutic benefits.
The “Research Chemical” Grey Area
While peptides genuinely used for laboratory research are exempt from drug regulations, companies selling peptides intended for human use under the guise of research chemicals risk FDA enforcement.
FDA Enforcement Actions
- Warning letters for unapproved or misbranded products
- Injunctions and product seizures
- Criminal prosecutions in severe cases
Approved Therapeutic Peptides
More than 80 peptide drugs have been approved by the FDA, including insulin, GLP-1 agonists, octreotide, vasopressin analogs, and calcitonin. These drugs are available only by prescription and manufactured under strict quality standards.
DEA Scheduling and Controlled Substances
Generally Unscheduled
Most peptides are not controlled substances under the Controlled Substances Act because they lack significant abuse potential.
Exceptions and Designer Peptides
Peptides related to controlled substances or designed to mimic their effects may face scrutiny under the Federal Analog Act or emergency scheduling provisions.
State-Level Regulations
- State pharmacy laws may classify peptides as prescription-only
- Some states apply broader controlled substance definitions
- Healthcare providers must comply with state licensing and scope-of-practice rules
- Anti-doping laws may prohibit certain peptides
International Regulatory Perspectives
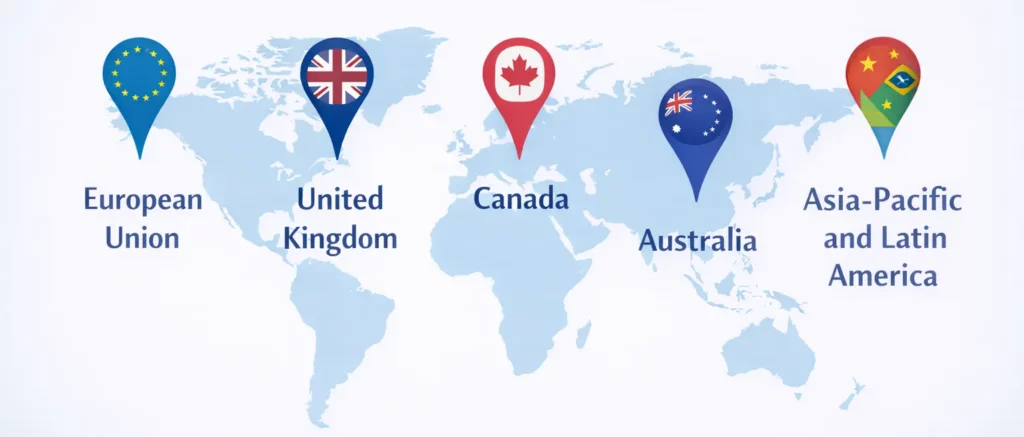
European Union
The European Medicines Agency (EMA) regulates therapeutic peptides, requiring marketing authorization and pharmacovigilance compliance.
United Kingdom
The MHRA oversees peptide regulation post-Brexit, following frameworks similar to EU standards.
Canada
Health Canada regulates peptides as drugs when intended for therapeutic use.
Australia
The Therapeutic Goods Administration (TGA) strictly controls peptide importation and therapeutic use.
Asia-Pacific and Latin America
Regulatory rigor varies widely, ranging from highly structured systems to loosely enforced frameworks.
Anti-Doping and Athletic Regulations
WADA Prohibited List
The World Anti-Doping Agency bans many peptides, including growth hormone secretagogues and growth factors.
Professional and Collegiate Sports
Professional leagues and collegiate organizations enforce strict peptide prohibitions with severe penalties for violations.
Customs and Import/Export Regulations
Import Restrictions
Many countries restrict peptide imports without licenses or documentation. Seizures and penalties are common.
Personal Import Risks
Personal importation of peptides often violates customs laws, even when labeled for research use.
Compounding Pharmacy Considerations
Legal Compounding
Licensed compounding pharmacies may prepare peptides with valid prescriptions, compliant APIs, and adherence to USP and FDA regulations.
503A vs 503B Facilities
503A pharmacies compound patient-specific prescriptions, while 503B outsourcing facilities operate under stricter FDA oversight.
Online Peptide Marketplaces
Legitimate Suppliers
Operate transparently, avoid therapeutic claims, and serve verified research institutions.
Grey Market and Illegal Sellers
Many online vendors mislabel peptides, make health claims, or sell substandard products, exposing buyers to legal and safety risks.
Individual Legal Risks
- Possession may violate drug or pharmacy laws
- Import violations can lead to seizure and penalties
- Fraudulent acquisition increases legal exposure
- Lack of medical oversight increases liability
Healthcare Provider Legal Responsibilities
Scope of Practice and Standard of Care
Providers must prescribe within licensed authority and meet professional standards.
Unapproved and Compounded Peptides
Prescribing unapproved peptides is not equivalent to off-label use and carries substantial legal risk.
Research and Institutional Compliance
IRB and IACUC Approval
Human and animal research requires institutional oversight and ethical review.
Institutional and Funding Compliance
Researchers must follow institutional policies and funding agency requirements.
Best Practices for Compliance
- Understand all applicable regulations
- Use reputable, compliant suppliers
- Maintain thorough documentation
- Consult legal and compliance experts
- Monitor regulatory developments
- Prioritize safety and quality
Conclusion
The regulatory environment for research peptides is multifaceted and continuously evolving. While legitimate research and therapeutic uses are legally supported, grey areas create significant risks for non-compliant actors.
Understanding and following applicable regulations is essential for legal protection, safety, and ethical conduct. As peptide science advances, regulatory oversight will likely increase, making compliance and transparency more important than ever.
- Peptides for Weight Loss: What Current Research Suggests - February 18, 2026
- IV Hydration Therapy: Benefits, Safety, and When It’s Most Effective - February 4, 2026
- The Future of Peptide Research: Emerging Trends and Innovations - January 30, 2026