PT-141, also known as bremelanotide, represents a unique peptide that has carved its niche in therapeutic applications, particularly in sexual health. As a melanocortin receptor agonist, PT-141 works through novel mechanisms distinct from traditional approaches, offering insights into complex physiological systems involving appetite, sexual function, inflammation, and more. This comprehensive guide explores PT-141’s science, applications, research status, and broader implications for melanocortin biology.
Understanding PT-141’s Origins and Development
PT-141’s story begins with melanotan II, a synthetic analog of alpha-melanocyte-stimulating hormone (α-MSH), a naturally occurring peptide hormone. Melanotan II was originally developed to stimulate melanin production for tanning purposes, potentially reducing skin cancer risk by providing darker pigmentation without sun exposure.
During melanotan II research, an unexpected side effect emerged: many subjects reported enhanced sexual arousal and function. This serendipitous discovery led researchers to explore sexual function applications more deliberately, eventually developing PT-141 as a modified version optimized for this purpose while reducing unwanted effects.
The “PT” designation refers to Palatin Technologies, the pharmaceutical company that developed the compound, while “141” represents the compound number in their development pipeline. The generic name bremelanotide reflects its chemical structure and classification.
Unlike phosphodiesterase-5 (PDE5) inhibitors like sildenafil (Viagra), tadalafil (Cialis), or vardenafil (Levitra) which work primarily on vascular mechanisms, PT-141 acts through the central nervous system, representing a fundamentally different approach to sexual function.
Melanocortin Receptors and PT-141’s Mechanism
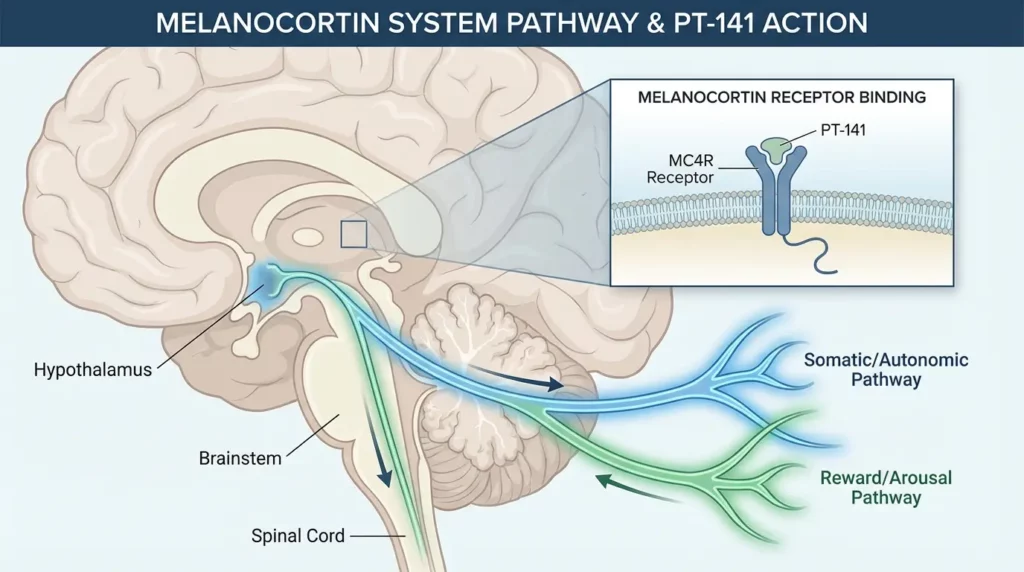
PT-141’s effects stem from its interaction with melanocortin receptors, a family of five G-protein coupled receptors (MC1R through MC5R) involved in diverse physiological processes.
The Melanocortin System
Melanocortin receptors respond to peptides derived from pro-opiomelanocortin (POMC), a precursor protein processed into several bioactive peptides including α-MSH, β-MSH, γ-MSH, and ACTH (adrenocorticotropic hormone). These peptides regulate pigmentation (MC1R), adrenal function (MC2R), energy homeostasis and inflammation (MC3R), appetite and metabolism (MC4R), and various functions including exocrine glands and possibly sexual function (MC5R).
PT-141’s Receptor Activity
PT-141 acts primarily as a melanocortin-4 receptor (MC4R) agonist, though it shows activity at other melanocortin receptors. The MC4R is expressed widely in the brain, particularly in regions involved in appetite regulation, energy balance, and sexual behavior. By activating MC4R, PT-141 influences neural circuits controlling sexual arousal and desire, working through the central nervous system rather than peripheral vascular mechanisms.
This central action distinguishes PT-141 from PDE5 inhibitors. While PDE5 inhibitors enhance blood flow to facilitate erectile response to sexual stimulation, PT-141 influences the desire and arousal components of sexual function at the level of brain signaling. This fundamental difference explains why PT-141 may benefit individuals who don’t respond to PDE5 inhibitors and why it’s particularly relevant for hypoactive sexual desire disorder.
Sexual Function Applications
PT-141’s primary studied application involves sexual dysfunction, where it received FDA approval for a specific indication.
Hypoactive Sexual Desire Disorder (HSDD) in Women
HSDD, characterized by persistently low sexual desire causing distress, affects a substantial proportion of women at some point in their lives. Traditional treatments have been limited, with psychological interventions, hormone therapy, and off-label medications showing variable efficacy.
PT-141 received FDA approval in 2019 (marketed as Vyleesi) specifically for treating acquired, generalized HSDD in premenopausal women. Clinical trials demonstrated that PT-141 significantly improved sexual desire compared to placebo, increased satisfying sexual events, and improved distress related to low sexual desire.
The medication is administered as a subcutaneous injection at least 45 minutes before anticipated sexual activity, acting within 30-60 minutes and maintaining effects for several hours. This on-demand dosing differs from daily medications, potentially appealing to women who prefer episodic treatment.
Erectile Dysfunction Research
Though not FDA-approved for male erectile dysfunction, PT-141 has been studied in this population. Research suggests potential benefits for men with erectile dysfunction, particularly those with psychogenic erectile dysfunction where psychological factors predominate, situations where PDE5 inhibitors are ineffective or contraindicated, and potentially as an adjunct to other treatments.
PT-141’s central mechanism might particularly benefit erectile dysfunction with strong psychological components, as it may enhance sexual desire and arousal at the neural level rather than merely facilitating mechanical erectile response.
Sexual Arousal Disorder
Beyond desire, sexual arousal itself involves complex neural, vascular, and hormonal components. Research explores whether PT-141’s effects extend to sexual arousal disorder, where individuals lack the physiological and psychological responses associated with sexual excitement.
Early research suggests PT-141 may enhance subjective arousal, improve physiological arousal markers, increase sexual satisfaction, and potentially benefit both men and women with arousal difficulties.
Mechanisms Beyond Sexual Function
The melanocortin system’s broad physiological roles mean PT-141 potentially influences processes beyond sexual function, creating research interest in additional applications.
Appetite and Metabolism
MC4R plays crucial roles in appetite regulation and energy balance. MC4R agonism generally reduces food intake and increases energy expenditure. PT-141, as an MC4R agonist, theoretically might influence these processes, potentially offering metabolic benefits.
However, weight loss and appetite suppression are not emphasized in PT-141’s approved indication, and the compound’s dosing and administration differ substantially from what might be optimal for metabolic applications. Still, understanding MC4R’s metabolic roles provides broader context for PT-141’s pharmacology.
Anti-Inflammatory Effects
Melanocortin peptides, particularly α-MSH, possess anti-inflammatory properties mediated through melanocortin receptors including MC3R and MC4R. These anti-inflammatory effects involve reducing pro-inflammatory cytokine production, modulating immune cell activity, protecting tissues from inflammatory damage, and potentially promoting resolution of inflammation.
Research has explored whether melanocortin agonists including PT-141 might have therapeutic applications in inflammatory conditions. While this remains largely preclinical research, the melanocortin system’s anti-inflammatory roles represent an intriguing area for future investigation.
Neuroprotection
Some research suggests melanocortin system activation might offer neuroprotective benefits through reducing neuroinflammation, supporting neuronal survival, modulating oxidative stress, and potentially improving outcomes in neurological injuries or diseases.
PT-141’s ability to cross the blood-brain barrier and activate central melanocortin receptors makes it theoretically interesting for neuroprotection research, though this remains highly speculative and would require substantial investigation before any clinical applications.
Administration and Pharmacokinetics
PT-141’s approved formulation involves subcutaneous injection using a pre-filled autoinjector pen, similar to those used for many other injectable medications. The standard dose is 1.75 mg administered subcutaneously in the abdomen or thigh.
Pharmacokinetic Profile
After subcutaneous injection, PT-141 is absorbed over about 30-60 minutes, reaching peak plasma concentrations approximately one hour after administration. Effects on sexual desire and arousal may begin within 30 minutes to 1 hour and persist for several hours, though timing varies individually.
The peptide undergoes metabolism and excretion with a half-life of approximately 2.7 hours, meaning the compound clears from the body relatively quickly. This short half-life supports the on-demand dosing strategy rather than requiring daily administration.
Dosing Considerations
The FDA-approved dosing involves using PT-141 no more than eight times per month and no more than once within 24 hours. This dosing limitation stems from safety considerations including nausea (a common side effect) and theoretical concerns about melanocortin receptor desensitization with very frequent use.
For research purposes, dosing protocols vary depending on the specific application being investigated. Understanding proper reconstitution (if using lyophilized powder), storage (typically refrigerated), and administration technique is crucial for both efficacy and safety.
Side Effects and Safety Profile
Clinical trials and post-marketing experience have established PT-141’s safety profile, with several noteworthy side effects that users should understand.
Common Side Effects
The most frequently reported side effects include nausea (affecting approximately 40% of users in clinical trials, typically transient and often diminishing with repeated use), flushing (facial redness and warmth), injection site reactions (pain, redness, or irritation at the injection site), headache, and vomiting in some cases.
Nausea represents the most significant tolerability issue. It typically begins 30-60 minutes after injection, may last several hours, and often improves with repeated use as tolerance develops. Anti-nausea strategies including taking with food (though food may slightly delay absorption), using anti-nausea medication prophylactically, ensuring adequate hydration, and starting with the lowest effective dose can help manage this side effect.
Cardiovascular Effects
PT-141 can cause transient increases in blood pressure and heart rate, typically modest and lasting only hours. However, these effects necessitate caution in individuals with cardiovascular disease, uncontrolled hypertension, or recent cardiovascular events.
Blood pressure should be measured before and after administration, particularly during initial uses. Individuals with cardiovascular risk factors should consult healthcare providers before using PT-141.
Skin Pigmentation
As a melanocortin agonist related to melanotan II, PT-141 might theoretically affect melanin production and skin pigmentation. However, this effect is minimal with PT-141 at therapeutic doses for sexual function, particularly compared to melanotan II. Most users don’t experience noticeable pigmentation changes.
Contraindications
PT-141 is contraindicated in uncontrolled hypertension or cardiovascular disease, pregnancy and breastfeeding (due to limited safety data), and known hypersensitivity to the medication or its components.
Drug Interactions
Limited drug interaction data exists for PT-141. Potential concerns include medications affecting blood pressure (combined hypotensive or hypertensive effects), drugs metabolized through similar pathways, and medications affecting the melanocortin system.
Clinical Trial Results and Efficacy
The FDA approval of PT-141 for HSDD in premenopausal women relied on pivotal clinical trials demonstrating efficacy and safety.
HSDD Trial Results
Two randomized, double-blind, placebo-controlled Phase 3 trials enrolled over 1,200 premenopausal women with HSDD. Primary endpoints included change in desire (measured by validated questionnaires), change in satisfying sexual events, and reduction in distress related to low sexual desire.
Results showed statistically significant improvements in all three primary endpoints compared to placebo. Women using PT-141 reported meaningful increases in sexual desire, more satisfying sexual events per month, and reduced distress about their low sexual desire.
Effect sizes were moderate, with about one additional satisfying sexual event per month compared to placebo. While this might seem modest, for women distressed by HSDD, this represents a clinically meaningful improvement. Patient-reported outcomes suggested many women found the treatment beneficial despite numerical effect sizes.
Male Erectile Dysfunction Studies
While not FDA-approved for this indication, studies in men with erectile dysfunction showed PT-141 improved erectile function in some participants, particularly those with psychogenic erectile dysfunction. Response rates varied, with some men experiencing substantial benefits while others showed little response.
The heterogeneous response suggests patient selection matters—identifying which men are most likely to benefit from PT-141’s mechanism could optimize outcomes.
Comparing PT-141 to Other Sexual Function Treatments
Understanding how PT-141 compares to alternative treatments helps contextualize its role in sexual health.
PT-141 vs. PDE5 Inhibitors
PDE5 inhibitors (Viagra, Cialis, etc.) represent first-line treatment for erectile dysfunction in men and have been explored for female sexual dysfunction. Key differences include mechanism (PT-141 acts centrally on desire/arousal circuits; PDE5 inhibitors act peripherally on vasculature), administration (PT-141 injection vs. PDE5 inhibitors oral), timing (PT-141 45+ minutes before, lasting hours; PDE5 inhibitors vary from 30 minutes to multiple days depending on specific drug), side effects (different profiles with PT-141’s nausea vs. PDE5 inhibitors’ headaches, flushing, visual changes), and indications (PT-141 FDA-approved for female HSDD; PDE5 inhibitors FDA-approved for male ED).
These differences mean the treatments may benefit different patient populations or potentially work synergistically.
PT-141 vs. Flibanserin
Flibanserin (Addyi) is another FDA-approved treatment for HSDD in premenopausal women, working through serotonin and dopamine pathways. Comparing the two reveals different mechanisms (PT-141 melanocortin agonist vs. flibanserin serotonin/dopamine modulator), administration (PT-141 on-demand injection vs. flibanserin daily oral), side effects (distinct profiles), and efficacy (both show modest but meaningful benefits with variable individual responses).
Choice between these options depends on patient preferences, tolerability, and individual response patterns.
Research into Melanocortin Biology
PT-141’s development and use have contributed to broader understanding of melanocortin biology and opened avenues for additional research.
Melanocortin Receptor Subtypes
Understanding specific roles of different melanocortin receptor subtypes continues advancing. MC4R’s role in sexual function, appetite, and metabolism makes it a particularly interesting therapeutic target. Developing compounds with selective activity at specific melanocortin receptor subtypes might enable more targeted therapies with improved side effect profiles.
Central vs. Peripheral Actions
Melanocortin receptors exist both in the brain and peripheral tissues. Distinguishing central from peripheral contributions to PT-141’s effects helps understand its mechanisms and might inform development of compounds optimized for specific applications.
Sexual Behavior Neuroscience
PT-141 research has contributed to understanding neural circuits underlying sexual desire, arousal, and behavior. Identifying brain regions and pathways responsive to melanocortin agonists illuminates the neuroscience of sexual function, potentially revealing additional therapeutic targets.
Practical Considerations for Use
For individuals considering PT-141, several practical factors merit consideration:
Patient Selection
PT-141 appears most beneficial for individuals with genuine desire disorders causing distress, particularly women with HSDD. It may be less effective for sexual dysfunction primarily due to relationship issues, physical factors like atrophy or pain, medication side effects from other drugs, or other specific medical causes.
Appropriate screening and evaluation help identify individuals most likely to benefit while avoiding use in those unlikely to respond or at higher risk of side effects.
Expectations and Response
Setting realistic expectations is important. PT-141 provides modest to moderate benefits for many users rather than dramatic transformative effects. Individual responses vary substantially, with some experiencing significant benefits while others notice minimal effects. The treatment addresses one component of sexual function (desire/arousal) but doesn’t solve all sexual health challenges. Effects are temporary and require repeated dosing for ongoing benefits.
Integration with Other Interventions
PT-141 works best as part of comprehensive sexual health approaches including relationship counseling when relevant, addressing underlying medical or psychological factors, optimizing general health and wellness, and considering complementary treatments for other sexual dysfunction components.
Cost and Access
PT-141’s cost varies depending on insurance coverage, pharmacy, and location. Without insurance, the medication can be expensive, potentially limiting access. Some insurance plans cover PT-141 for its FDA-approved indication, while others don’t. Research applications and off-label uses typically aren’t covered by insurance, requiring out-of-pocket payment.
Future Research Directions
Ongoing and future research will further clarify PT-141’s role and explore additional applications:
- Long-term efficacy and safety beyond the duration of clinical trials
- Optimization of dosing and administration to improve tolerability
- Identification of biomarkers predicting response
- Exploration of combination approaches with other sexual function treatments
- Investigation of applications beyond sexual function where melanocortin agonism might benefit health
- Development of next-generation melanocortin agonists with improved properties
Conclusion
PT-141 represents an innovative approach to sexual function, working through melanocortin receptors in the central nervous system to enhance sexual desire and arousal. Its FDA approval for HSDD in premenopausal women validates this mechanism and provides a new option for a condition with limited treatment alternatives.
Beyond sexual function, PT-141 illuminates the broader biology of melanocortin systems and their roles in appetite, metabolism, inflammation, and other processes. Whether these additional applications translate to therapeutic uses remains to be determined by future research.
For individuals dealing with sexual desire disorders, PT-141 offers a treatment option with a unique mechanism, distinct from existing alternatives. While not perfect—with side effects like nausea and moderate efficacy—it provides meaningful benefits for many users who previously had limited options.
As research continues and our understanding of melanocortin biology deepens, PT-141 and related compounds may reveal additional applications, contributing to sexual health and potentially broader aspects of human physiology and medicine.
- Peptides for Weight Loss: What Current Research Suggests - February 18, 2026
- IV Hydration Therapy: Benefits, Safety, and When It’s Most Effective - February 4, 2026
- The Future of Peptide Research: Emerging Trends and Innovations - January 30, 2026