deepening biological understanding, and expanding therapeutic applications creating unprecedented opportunities.
From artificial intelligence revolutionizing peptide design to novel delivery systems overcoming traditional
limitations, the future of peptide science promises transformative advances in medicine, biotechnology, and beyond.
This comprehensive exploration examines emerging trends, breakthrough technologies, and future directions that
will shape peptide research in the coming decades.
Artificial Intelligence and Computational Peptide Design
Perhaps no development promises to accelerate peptide research more dramatically than artificial intelligence
and machine learning applications.
AI-Driven Peptide Discovery
Traditional peptide discovery involved laborious screening of vast libraries. AI predicts peptide properties
from sequence, including binding affinity, stability, solubility, toxicity, and pharmacokinetic parameters.
Machine learning models can generate novel sequences optimized for specific properties, reducing time and cost
of lead identification from months to days or hours.
Deep Learning for Structure Prediction
AlphaFold and similar systems have transformed protein structure prediction, extending to peptides.
Understanding three-dimensional structure enables better function prediction, guides modifications,
and supports rational design approaches.
Generative Models
GANs and VAEs generate entirely novel peptide sequences with desired properties, maintaining beneficial
features while exploring new chemical space. This unlocks therapeutic applications not previously conceived.
Integration with High-Throughput Screening
Combining AI prediction with automated synthesis and high-throughput screening accelerates optimization
exponentially, creating a closed-loop discovery cycle.
Novel Peptide Modifications and Chemistry
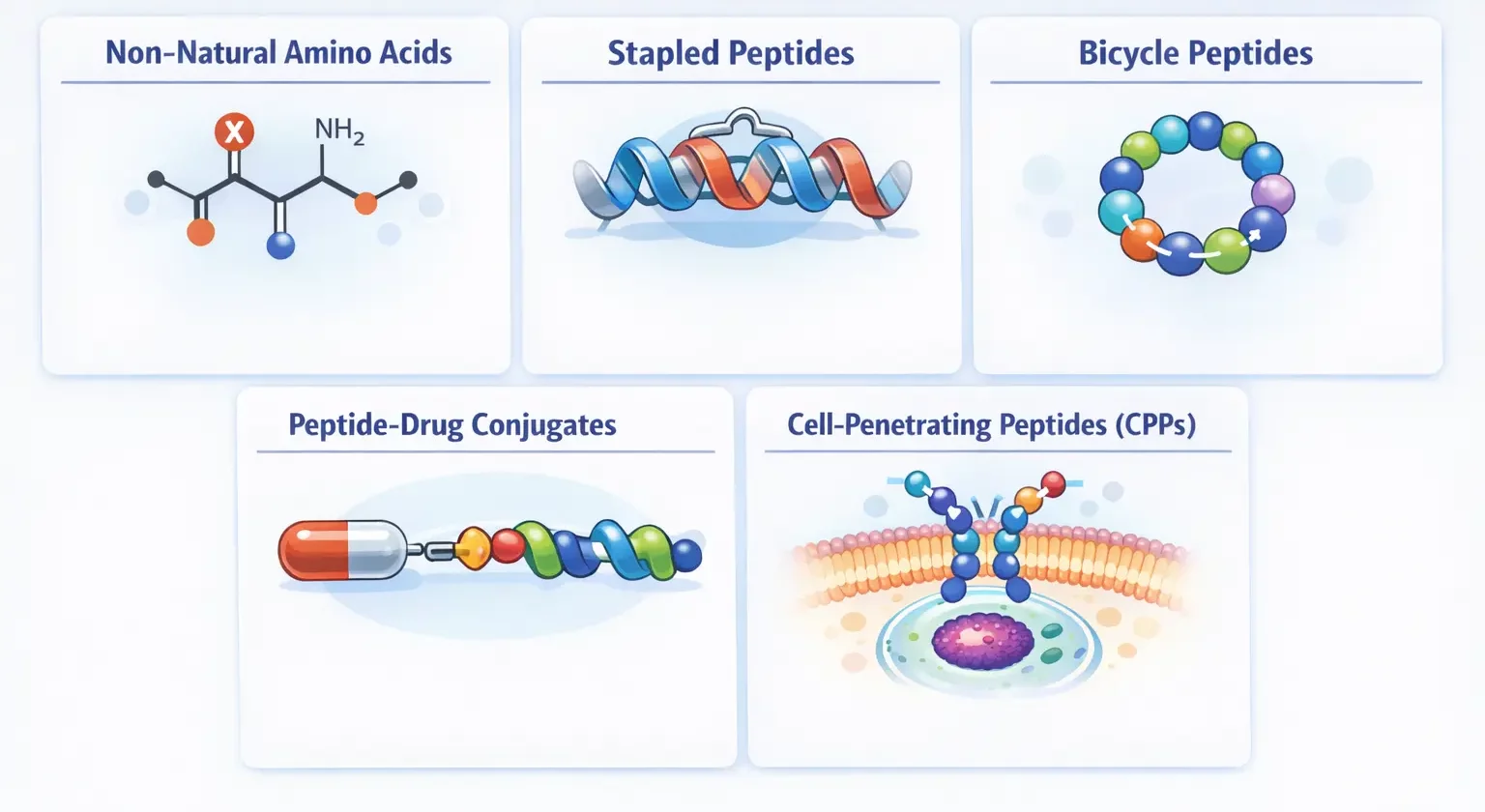
Non-Natural Amino Acids
Incorporating amino acids not found in nature provides enhanced protease resistance, novel functionalities,
structural rigidity, and altered properties. These modifications produce designer peptides with optimized
therapeutic profiles.
Stapled Peptides
Chemically linking non-adjacent amino acids stabilizes helical structures, enhancing protease resistance,
cell penetration, binding affinity, and pharmacokinetics.
Bicycle Peptides
Peptides with two constrained loops offer exceptional target specificity, stability, and protease resistance,
useful for tumor targeting and imaging.
Peptide-Drug Conjugates
PDCs combine targeting peptides with cytotoxic payloads, minimizing systemic toxicity and enhancing
targeted delivery. They offer improved tissue penetration and reduced immunogenicity compared to
antibody-drug conjugates.
Cell-Penetrating Peptides (CPPs)
CPPs facilitate cellular uptake of attached cargos, enabling delivery of otherwise cell-impermeable compounds,
drugs, nucleic acids, or proteins.
Revolutionary Delivery Systems
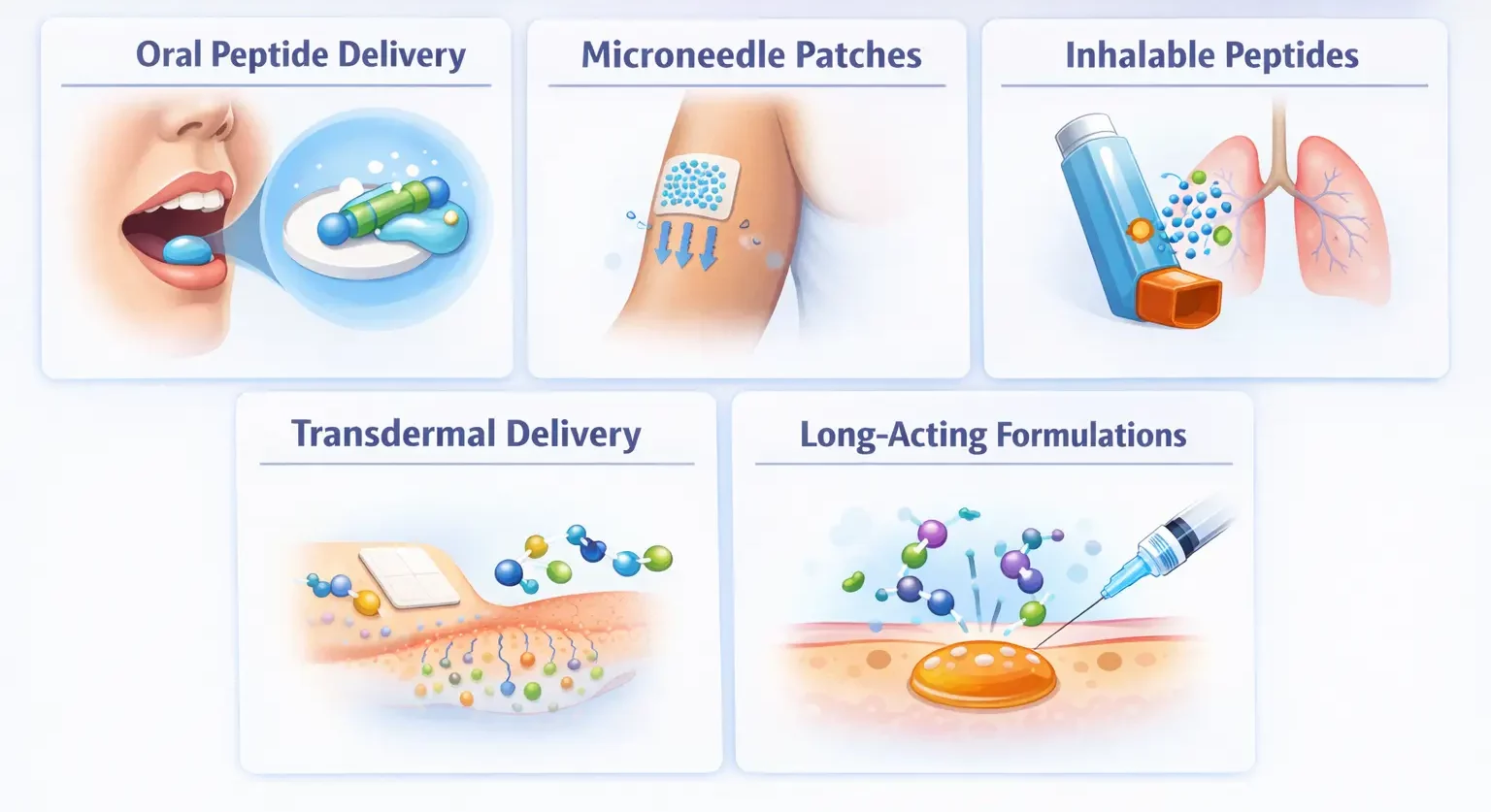
Oral Peptide Delivery
Oral bioavailability is achieved via absorption enhancers, protease inhibitors, and nanoparticle encapsulation.
FDA-approved oral semaglutide uses absorption enhancer SNAC.
Microneedle Patches
Pain-free patches deliver peptides through skin with steady release kinetics, improved compliance,
and potential at-home administration.
Inhalable Peptides
Pulmonary delivery allows rapid absorption and direct lung treatment. Dry powder and aerosol formulations
enhance viability.
Transdermal Delivery
Techniques include iontophoresis, ultrasound, chemical enhancers, and nanocarriers for peptide transport through skin.
Long-Acting Formulations
Depot injections, PEGylation, implantable devices, and carrier fusion extend peptide half-life, reducing
dosing frequency.
Expanding Therapeutic Applications
Oncology Applications
Tumor-targeting peptides, immunomodulatory peptides, and cancer vaccine peptides enhance diagnostics
and targeted therapy.
Antimicrobial Peptides (AMPs)
AMPs offer broad-spectrum activity, reduced resistance potential, topical/systemic applications,
and immunomodulatory effects.
Metabolic Disease
Multi-agonist peptides, mitochondrial-targeted peptides, and modulators of metabolic hormones target
diabetes, obesity, and insulin resistance.
Neurodegenerative Diseases
Neuroprotective peptides, aggregation inhibitors, neurotrophic mimetics, and peptides enhancing
neuroplasticity support brain health.
Regenerative Medicine
Growth factor, matrix, angiogenic, and anti-fibrotic peptides promote tissue regeneration and repair.
Cardiovascular Disease
Natriuretic, vasoactive, anti-thrombotic, and cardioprotective peptides address heart and vascular conditions.
Peptide-Based Diagnostics and Imaging
Molecular Imaging
Peptides labeled with radioactive or fluorescent markers enable targeted imaging of tissues or disease,
particularly in cancer, inflammation, and cardiovascular disease.
Biosensors
Peptide-based biosensors detect molecules, monitor biomarkers, and measure drug levels or metabolites.
Companion Diagnostics
Peptides help identify patients likely to respond to specific therapies, enabling personalized medicine.
Gene and Cell Therapy Applications
Cell-Penetrating Peptides for Gene Delivery
CPPs deliver nucleic acids into cells without viral vectors, enabling non-viral gene therapy and CRISPR delivery.
Peptide-Mediated Genome Editing
Peptides fused to genome-editing enzymes improve targeting and uptake, enhancing specificity and efficiency.
CAR-T Cell Engineering
Peptides enhance tumor targeting, modulate CAR-T activity, and improve persistence and efficacy.
Personalized and Precision Peptide Medicine
Patient-Specific Peptides
Peptides tailored to individual genetics, tumor mutations, and pharmacokinetics enable precision therapy.
Biomarker-Guided Therapy
Biomarkers predict peptide response, guide dosing, and monitor effectiveness.
3D Bioprinting with Peptide Hydrogels
Peptide-based hydrogels serve as bioinks for patient-specific tissue and organ printing.
Manufacturing and Scale-Up Innovations
Continuous Flow Synthesis
Offers faster synthesis, easier scaling, better quality control, and reduced costs.
Enzymatic Synthesis
Greener chemistry with selective reactions and reduced waste for large-scale production.
Cell-Free Protein Synthesis
Rapid production of peptides and small proteins without living cells.
Recombinant Production
Genetically engineered cells produce peptides affordably at commercial scale.
Regulatory and Commercialization Landscape
Streamlined Regulatory Pathways
Specialized guidance accelerates approval and clarifies requirements for peptide therapeutics.
Biosimilar/Generic Peptides
Emerging pathways reduce costs and expand access to established therapies.
Academic-Industry Partnerships
Collaboration accelerates translation from discovery to commercialization.
Venture Investment
Funding supports translational research and clinical development.
Challenges and Limitations to Address
Immunogenicity
Some peptides trigger immune responses; strategies are needed to reduce immunogenicity.
Stability
Many peptides remain less stable than small molecules; continued stabilization innovations are required.
Manufacturing Costs
Complex peptides are expensive to produce; cost reduction remains a priority.
Delivery to Intracellular Targets
Efficient intracellular delivery remains challenging despite CPP advances.
Intellectual Property Complexity
Peptide patents are complex; careful navigation is needed to protect innovations.
Interdisciplinary Integration
Leading peptide research integrates chemistry, biology, computational science, medicine, engineering, academia, and industry.
Educational and Workforce Implications
Academic Programs
Universities develop specialized curricula, graduate programs, and continuing education in peptide science.
Industry Training
Companies build internal expertise and partner with academia for training.
Professional Societies
Societies provide knowledge sharing, conferences, workshops, and training.
Timeline and Predictions
Near-Term (2-5 years)
Approvals of novel therapeutics, AI adoption, improved delivery systems, multi-agonist peptides,
antimicrobial applications.
Medium-Term (5-10 years)
Personalized peptide therapeutics, cancer vaccines, advanced brain delivery, regenerative medicine,
biosimilars.
Long-Term (10+ years)
Fully AI-designed therapeutics, treatments for untreatable conditions, integration with gene and cell therapy,
aging interventions, peptides as a dominant modality.
Conclusion
The future of peptide research is bright, driven by technological advances, novel chemistries, breakthrough delivery
systems, and expanding applications. Peptides promise more potent, specific, and accessible therapeutics, transforming
medicine and improving human health.
- Peptides for Weight Loss: What Current Research Suggests - February 18, 2026
- IV Hydration Therapy: Benefits, Safety, and When It’s Most Effective - February 4, 2026
- The Future of Peptide Research: Emerging Trends and Innovations - January 30, 2026