Cerebrolysin represents a unique neuroprotective and neurotrophic peptide preparation that has been researched for over five decades in neurological conditions. Derived from porcine brain tissue, this complex mixture of bioactive peptides mimics the actions of naturally occurring neurotrophic factors, supporting neuronal survival, promoting neuroplasticity, and potentially enhancing cognitive function. This comprehensive guide explores Cerebrolysin’s composition, mechanisms, research applications, and what current evidence reveals about its potential in neurological health.
Understanding Cerebrolysin’s Composition

Unlike single-molecule peptides, Cerebrolysin is a sophisticated mixture of low-molecular-weight peptides and amino acids derived through enzymatic breakdown of purified porcine brain proteins. This complex composition includes peptide fragments that share structural and functional similarities with endogenous neurotrophic factors—proteins that support the growth, survival, and differentiation of neurons.
The preparation contains multiple bioactive components including brain-derived neurotrophic factor (BDNF)-like peptides, nerve growth factor (NGF)-like peptides, ciliary neurotrophic factor (CNTF)-like peptides, and glial cell line-derived neurotrophic factor (GDNF)-like peptides. This multimodal composition may explain Cerebrolysin’s diverse neuroprotective and neurotrophic effects, as it potentially activates multiple signaling pathways simultaneously.
The manufacturing process involves careful selection of source material, enzymatic processing to create specific peptide fragments, purification to remove unwanted components, standardization to ensure consistent composition, and rigorous quality control including testing for infectious agents and contaminants. This sophisticated production process aims to create a consistent, safe product that retains biological activity.
Mechanisms of Neuroprotection
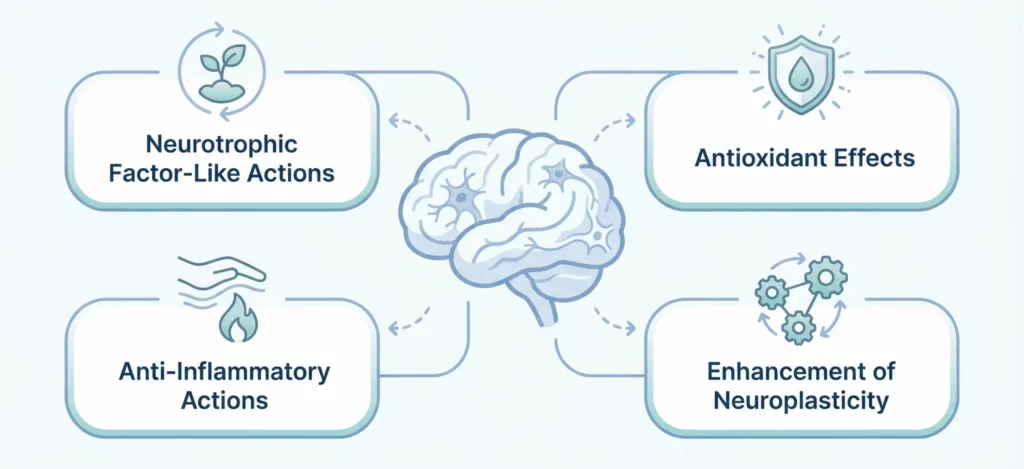
Cerebrolysin’s neuroprotective mechanisms are multifaceted, working through several complementary pathways to protect neurons from injury and support recovery.
Neurotrophic Factor-Like Actions
The peptides in Cerebrolysin activate intracellular signaling pathways similar to those triggered by natural neurotrophic factors. These pathways promote neuronal survival by suppressing apoptosis (programmed cell death), enhance neuronal metabolism and energy production, support synaptic function and plasticity, and promote neurite outgrowth—the extension of neuronal processes that form connections with other neurons.
Antioxidant Effects
Oxidative stress—excessive production of reactive oxygen species that damage cellular components—plays a major role in neurological injury and degeneration. Cerebrolysin appears to reduce oxidative stress through multiple mechanisms, including enhancing endogenous antioxidant systems, reducing free radical production, protecting mitochondrial function (the energy-producing organelles particularly vulnerable to oxidative damage), and stabilizing cellular membranes against oxidative injury.
Anti-Inflammatory Actions
Neuroinflammation contributes to neuronal damage in acute injuries like stroke and traumatic brain injury, as well as chronic neurodegenerative conditions. Cerebrolysin modulates inflammatory responses by reducing pro-inflammatory cytokine production, limiting microglial activation (microglia are brain immune cells that can cause damage when overactivated), protecting the blood-brain barrier from inflammatory disruption, and promoting resolution of inflammation.
Enhancement of Neuroplasticity
Perhaps most importantly for recovery from neurological injury, Cerebrolysin appears to enhance neuroplasticity—the brain’s ability to reorganize and form new neural connections. This occurs through promoting synaptogenesis (formation of new synapses), supporting neurogenesis (generation of new neurons in certain brain regions), enhancing long-term potentiation (a cellular mechanism underlying learning and memory), and facilitating functional reorganization after injury.
Research in Stroke and Cerebrovascular Disease
Stroke, caused by disrupted blood flow to brain tissue, represents one of the most extensively studied applications for Cerebrolysin. Both ischemic stroke (caused by blood vessel blockage) and hemorrhagic stroke (caused by bleeding) result in neuronal death, inflammation, and functional deficits.
Acute Stroke Treatment
Research has explored Cerebrolysin as an adjunct to standard stroke care. The rationale is that while restoring blood flow (through clot-dissolving drugs or mechanical thrombectomy) is crucial, additional neuroprotective interventions might reduce the extent of brain damage and improve recovery.
Multiple clinical trials have investigated Cerebrolysin in acute ischemic stroke. A meta-analysis of these trials suggested potential benefits including improved functional outcomes measured by scales like the Modified Rankin Scale and Barthel Index, enhanced neurological recovery, better cognitive outcomes, and possibly reduced mortality, though results have been mixed and some studies showed limited or no benefit.
The timing of Cerebrolysin administration appears important. Research suggests early treatment (within hours to days of stroke onset) may be more effective than delayed administration, as it targets acute injury mechanisms and early recovery processes.
Stroke Recovery and Rehabilitation
Beyond the acute phase, Cerebrolysin has been studied during stroke rehabilitation. The hypothesis is that by enhancing neuroplasticity, Cerebrolysin might amplify the benefits of rehabilitation therapy, helping the brain reorganize and compensate for damaged areas.
Studies in stroke rehabilitation have examined whether Cerebrolysin combined with physical, occupational, and speech therapy produces better outcomes than rehabilitation alone. Some research suggests enhanced motor recovery, improved cognitive rehabilitation, better speech and language recovery in patients with aphasia, and accelerated overall functional improvement.
Vascular Cognitive Impairment
Chronic cerebrovascular disease can lead to vascular cognitive impairment and vascular dementia—cognitive decline resulting from cumulative damage to brain blood vessels. Cerebrolysin research has explored whether the peptide might slow cognitive decline or improve cognitive function in these conditions.
Traumatic Brain Injury Applications
Traumatic brain injury (TBI) causes both immediate mechanical damage and secondary injury processes including inflammation, excitotoxicity (excessive stimulation by neurotransmitters), oxidative stress, and progressive neurodegeneration. These secondary injury mechanisms represent potential therapeutic targets.
Acute TBI Management
Research has investigated Cerebrolysin as an adjunct to standard TBI care. Animal models of TBI show that Cerebrolysin can reduce lesion size, decrease brain swelling, protect neurons from secondary injury, improve behavioral outcomes, and enhance long-term functional recovery.
Human studies in TBI are more limited but suggest potential benefits. Some clinical trials have reported improved Glasgow Coma Scale scores (a measure of consciousness level), reduced duration of post-traumatic amnesia, better cognitive recovery, improved functional outcomes at hospital discharge and long-term follow-up, and potentially reduced post-concussive symptoms.
Chronic Effects of TBI
Even after apparent recovery from TBI, many patients experience persistent symptoms including cognitive difficulties, mood changes, fatigue, and increased risk of neurodegenerative conditions. Research is exploring whether Cerebrolysin might address these chronic effects by promoting ongoing neural repair, supporting cognitive function, reducing neuroinflammation, and potentially reducing long-term neurodegeneration risk.
Research in Neurodegenerative Diseases
Neurodegenerative conditions including Alzheimer’s disease, Parkinson’s disease, and others involve progressive loss of neurons and declining function. While these conditions differ in their specific pathology, they share common features including protein aggregation, oxidative stress, mitochondrial dysfunction, and neuroinflammation—processes that Cerebrolysin’s mechanisms might theoretically address.
Alzheimer’s Disease Research
Alzheimer’s disease, characterized by amyloid plaques, tau tangles, neuronal loss, and progressive cognitive decline, represents the most common cause of dementia. Cerebrolysin research in Alzheimer’s has explored whether the peptide preparation might slow disease progression or improve symptoms.
Clinical trials in Alzheimer’s disease have examined Cerebrolysin’s effects on cognitive function measured by tests like the Mini-Mental State Examination (MMSE) and Alzheimer’s Disease Assessment Scale-Cognitive subscale (ADAS-Cog), activities of daily living, behavioral and psychological symptoms, and biomarkers of neurodegeneration.
Results have been mixed. Some studies suggest modest improvements in cognitive function and daily activities, particularly in mild to moderate Alzheimer’s disease. The effects appear most pronounced when Cerebrolysin is used for extended periods (weeks to months) rather than brief courses. However, other studies have shown limited or no benefit, and the overall evidence quality has limitations including small sample sizes and methodological concerns.
Parkinson’s Disease
Parkinson’s disease involves loss of dopamine-producing neurons in the substantia nigra, leading to motor symptoms (tremor, rigidity, bradykinesia, postural instability) and non-motor features (cognitive impairment, depression, sleep disturbances). Research has explored whether Cerebrolysin might protect remaining dopamine neurons or improve symptoms.
Preclinical studies suggest Cerebrolysin might protect dopamine neurons from degeneration, reduce alpha-synuclein aggregation (the abnormal protein accumulation characteristic of Parkinson’s), support remaining neural networks, and potentially improve motor and non-motor symptoms.
Human studies are limited but suggest possible benefits in motor function, non-motor symptoms particularly cognitive impairment, and potentially slower disease progression, though more research is needed to establish these effects definitively.
Other Neurodegenerative Conditions
Research has also explored Cerebrolysin in conditions like amyotrophic lateral sclerosis (ALS), multiple system atrophy, and other neurodegenerative diseases, though evidence remains preliminary.
Cognitive Enhancement and Mild Cognitive Impairment
Beyond treating established neurological diseases, research has investigated whether Cerebrolysin might benefit individuals with mild cognitive impairment (MCI)—cognitive decline greater than expected for age but not meeting dementia criteria—or even enhance cognitive function in healthy aging.
Mild Cognitive Impairment
MCI represents a risk state for dementia, with approximately 10-15% of people with MCI progressing to dementia annually. Interventions that might prevent or delay this progression have enormous public health significance.
Studies in MCI have examined whether Cerebrolysin improves cognitive test scores, reduces progression to dementia, improves functional capacity, and enhances quality of life. Some research suggests potential benefits, particularly in memory and executive function domains, though the evidence base remains limited.
Age-Related Cognitive Decline
As people age, even without diagnosable cognitive impairment, most experience some cognitive changes including slower processing speed, reduced working memory capacity, decreased executive function, and subtler changes in memory. Research has explored whether Cerebrolysin might support cognitive function during normal aging.
Theoretical mechanisms include supporting synaptic health and plasticity, maintaining neurotrophic factor signaling, reducing age-related neuroinflammation and oxidative stress, supporting mitochondrial function in aging neurons, and promoting neural repair and maintenance processes.
Psychiatric and Mood Disorder Research
Emerging research explores Cerebrolysin’s potential in psychiatric conditions where neurotrophic factor deficiency and neuroplasticity impairment may play roles.
Depression
Major depressive disorder involves not only neurotransmitter imbalances but also reduced neurotrophic factor levels (particularly BDNF), decreased neuroplasticity, hippocampal volume loss, and neuroinflammation. Some research suggests neurotrophic interventions might offer antidepressant benefits.
Small studies have explored Cerebrolysin as an adjunct to standard antidepressant treatment, with some reporting enhanced antidepressant responses, faster symptom improvement, and improved treatment-resistant depression outcomes. However, this remains a highly preliminary research area requiring much more investigation.
Post-Stroke Depression
Depression commonly follows stroke, affecting 30-50% of survivors and negatively impacting rehabilitation and recovery. Research has examined whether Cerebrolysin’s effects on both stroke recovery and mood might make it particularly useful for post-stroke depression.
Developmental and Pediatric Applications
Some research has explored Cerebrolysin in pediatric neurological conditions including cerebral palsy, developmental delays, autism spectrum disorders, and learning disabilities. The rationale involves Cerebrolysin’s neurotrophic effects potentially supporting neurodevelopment and plasticity.
However, pediatric research is limited, and the risk-benefit balance in developing brains requires careful consideration. Most applications remain investigational, and Cerebrolysin is not routinely recommended for pediatric conditions outside research contexts.
Administration and Dosing
Cerebrolysin is administered via intravenous or intramuscular injection, as oral administration would result in peptide degradation in the digestive system. This administration requirement represents a practical limitation compared to oral medications.
Dosing Protocols
Dosing varies by condition and study protocol. Common approaches include daily doses of 10-60 mL administered intravenously, typically over 10-20 days, sometimes repeated in courses with intervals between, with maintenance protocols in chronic conditions.
Treatment Duration
Optimal treatment duration remains unclear and likely varies by condition. Acute conditions like stroke or TBI typically involve shorter courses (days to weeks), while chronic neurodegenerative conditions might require longer or repeated treatment courses.
Safety Profile and Side Effects
Cerebrolysin has been used clinically for decades, providing substantial safety data. The peptide preparation is generally well-tolerated, with serious adverse effects uncommon.
Common Side Effects
Most reported side effects are mild and include injection site reactions with intramuscular administration, occasional dizziness or headache, fatigue, mild gastrointestinal symptoms, and rarely, allergic reactions.
Contraindications
Cerebrolysin is contraindicated in epilepsy or seizure disorders (due to potential seizure threshold lowering), severe renal impairment, known allergy to porcine proteins, and acute severe kidney disease. Caution is advised in pregnancy and breastfeeding due to limited safety data.
Drug Interactions
Cerebrolysin may interact with monoamine oxidase inhibitors (MAOIs) and antidepressants, requiring careful monitoring. It should not be mixed with lipid-containing solutions or administered with certain other medications in the same infusion.
Quality and Sourcing Considerations
As with all biologically-derived peptide preparations, quality and sourcing are critical for Cerebrolysin. Legitimate pharmaceutical-grade Cerebrolysin undergoes rigorous manufacturing standards, testing for infectious agents including prions, standardization for consistent composition, proper storage requirements (typically refrigerated), and clear documentation of origin and testing.
Research applications require pharmaceutical-grade materials to ensure safety and reproducibility. Individuals interested in Cerebrolysin for research purposes should work with reputable suppliers and knowledgeable healthcare providers.
Current Evidence Quality and Limitations
While Cerebrolysin has been studied for decades, the overall evidence quality has limitations that must be acknowledged.
Study Quality Issues
Many Cerebrolysin studies suffer from small sample sizes, limited long-term follow-up, methodological concerns including inadequate blinding or randomization, heterogeneous patient populations making comparison difficult, and varying dosing protocols complicating meta-analyses.
Publication Bias
Concerns exist about publication bias, where positive studies are more likely to be published than negative studies, potentially overestimating benefits.
Mechanistic Questions
While general mechanisms are understood, many details about how Cerebrolysin’s complex peptide mixture produces effects remain unclear. Which specific peptides contribute which effects? How do the various components interact? What are the optimal formulations?
Comparative Effectiveness:
Limited research directly compares Cerebrolysin to other neuroprotective interventions, making it difficult to assess relative effectiveness.
These limitations don’t negate existing evidence but suggest cautious interpretation and highlight the need for higher-quality research.
Future Research Directions
Important research priorities for Cerebrolysin include larger, well-designed randomized controlled trials with adequate sample sizes and methodological rigor, identification of biomarkers predicting which patients will respond, exploration of optimal dosing and duration for different conditions, investigation of combination approaches with other neuroprotective or neurotrophic interventions, better mechanistic understanding of active components and their specific contributions, development of potentially improved formulations based on mechanistic insights, and exploration of novel applications where neurotrophic support might prove beneficial.
Practical Considerations
For individuals or practitioners interested in Cerebrolysin, several practical considerations apply:
Clinical Use
In countries where Cerebrolysin is approved, it’s typically used as an adjunct to standard neurological care rather than a standalone treatment. For conditions like stroke, standard interventions (clot removal, rehabilitation) remain primary, with Cerebrolysin potentially offering additional benefits.
Research Contexts
In research settings, Cerebrolysin use should follow appropriate protocols, include informed consent processes, involve appropriate safety monitoring, and be part of systematically designed studies contributing to evidence.
Regulatory Status
Cerebrolysin’s regulatory status varies globally. It’s approved for certain indications in some countries while unavailable or limited to research use in others. Understanding local regulatory status is essential.
Cost Considerations
Treatment courses involving daily injections for weeks can be costly. In regions without insurance coverage, this represents a significant access barrier.
Conclusion
Cerebrolysin represents an intriguing neuroprotective and neurotrophic peptide preparation with decades of research exploring diverse neurological applications. Its complex composition, multimodal mechanisms, and theoretical basis for promoting neural health and recovery make it appealing for conditions from acute brain injuries to chronic neurodegeneration.
Current evidence suggests potential benefits in several areas, particularly stroke, traumatic brain injury, and possibly some neurodegenerative conditions, though the quality and consistency of evidence varies. While not a miracle cure for neurological conditions, Cerebrolysin may offer a useful adjunctive tool in comprehensive neurological care.
As research continues and methodological quality improves, we’ll better understand where Cerebrolysin fits in the neurological therapeutic arsenal and which patients are most likely to benefit. For those dealing with neurological conditions or interested in neuroprotection, Cerebrolysin represents one option worth understanding as part of the broader landscape of neurological interventions.
The peptide’s long history of use, generally favorable safety profile, and theoretical mechanisms supporting neural health and recovery ensure it will remain an area of active research and clinical interest in neurological medicine.
- Peptides for Weight Loss: What Current Research Suggests - February 18, 2026
- IV Hydration Therapy: Benefits, Safety, and When It’s Most Effective - February 4, 2026
- The Future of Peptide Research: Emerging Trends and Innovations - January 30, 2026