Selecting quality research peptides is crucial for obtaining reliable, reproducible results in scientific research. The peptide market has expanded dramatically, with numerous suppliers offering products at varying quality levels and price points. For researchers, distinguishing high-quality peptides from inferior products requires understanding purity standards, testing methodologies, supplier credentials, and proper handling practices. This comprehensive guide provides the knowledge needed to make informed decisions when purchasing research peptides.
Why Peptide Quality Matters

The quality of research peptides directly impacts experimental outcomes, data reliability, and research integrity. Using substandard peptides can lead to inconsistent results that waste time and resources, false conclusions that mislead research directions, failed experiments requiring repetition, compromised data that may not be publishable, and potential safety issues in biological research.
Peptide quality encompasses several dimensions including purity (the percentage of the desired peptide versus impurities), identity (confirmation that the peptide is the correct sequence), stability (whether the peptide maintains integrity over time), and consistency (whether different batches show similar properties).
High-quality peptides undergo rigorous synthesis, purification, and testing to meet stringent specifications. Understanding what constitutes quality and how to verify it empowers researchers to select appropriate materials for their work.
Understanding Peptide Purity Grades
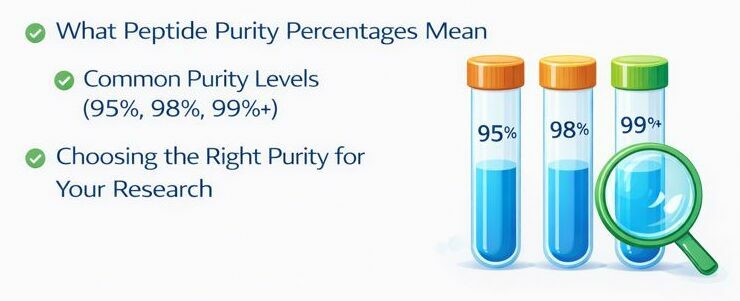
Peptide suppliers typically offer products at different purity grades, each suited for specific applications.
Research Grade (≥95% purity)
Research-grade peptides meet the needs of most basic and applied research applications. They undergo synthesis using solid-phase peptide synthesis (SPPS) or other methods, purification typically by high-performance liquid chromatography (HPLC), and analytical testing to confirm purity ≥95%.
This grade suits cell culture studies, in vitro biochemical assays, animal research where extremely high purity isn’t critical, and preliminary screening studies. The balance between quality and cost makes research-grade peptides the most commonly used category.
High Purity Grade (≥98% purity)
High-purity peptides undergo additional purification steps to achieve purity ≥98%, often approaching 99% or higher. These peptides are appropriate for studies requiring minimal impurities, sensitive biological assays, research leading toward clinical applications, and studies where even trace impurities might confound results.
The additional purification increases cost but provides higher confidence in data quality and reduces the risk of impurity-related artifacts.
Pharmaceutical Grade (≥99% purity)
Pharmaceutical-grade peptides meet the most stringent quality standards, including purity ≥99%, comprehensive testing including endotoxin levels, sterility testing when applicable, full regulatory documentation, and GMP (Good Manufacturing Practice) compliance.
These peptides are required for clinical trials, GLP (Good Laboratory Practice) toxicology studies, formulation development, and any research intended to support regulatory submissions. While most expensive, pharmaceutical-grade peptides are essential when regulatory compliance is necessary.
Crude/Unpurified Peptides (<80% purity)
Some suppliers offer crude peptides that have undergone synthesis but minimal or no purification. These products cost substantially less but contain significant impurities including truncated sequences, deletion peptides, side products from incomplete reactions, and residual synthesis reagents.
Crude peptides may suit very preliminary screening or large-scale applications where high purity isn’t critical, but most research applications require at least research-grade purity.
Essential Testing Methods and Certificates of Analysis

Quality peptide suppliers provide Certificates of Analysis (CoA) documenting comprehensive testing of each batch. Understanding common testing methods helps researchers interpret CoAs and verify quality claims.
High-Performance Liquid Chromatography (HPLC)
HPLC represents the primary method for assessing peptide purity. The technique separates compounds based on their chemical properties, producing a chromatogram showing peaks for different components.
A high-quality CoA includes an HPLC chromatogram showing a single dominant peak (the desired peptide) with minimal additional peaks (impurities), purity percentage calculated from peak areas, and analysis conditions (column type, mobile phase, detection wavelength).
Two HPLC variants provide complementary information: analytical HPLC confirms purity and is the standard reported method, while preparative HPLC is used for purification during manufacturing. Quality suppliers perform analytical HPLC after preparative purification to verify final purity.
Mass Spectrometry (MS)
Mass spectrometry confirms peptide identity by measuring molecular weight with high precision. The CoA should include expected molecular weight based on sequence, observed molecular weight from MS analysis, and acceptable variance (typically ±0.05% for high-quality analysis).
Different MS techniques offer various capabilities: MALDI-TOF (Matrix-Assisted Laser Desorption/Ionization Time-of-Flight) provides rapid molecular weight confirmation, ESI-MS (Electrospray Ionization Mass Spectrometry) offers high sensitivity and accuracy, and MS/MS can provide sequence confirmation through fragmentation analysis.
Amino Acid Analysis (AAA)
Amino acid analysis determines the actual amino acid composition, confirming the peptide sequence and providing quantitative information about peptide content.
AAA involves hydrolyzing the peptide into individual amino acids, separating and quantifying each amino acid, and comparing results to the expected composition. Deviations indicate synthesis errors or degradation.
While not always included in routine CoAs, AAA provides valuable confirmation for critical applications or when questions arise about peptide identity or content.
Peptide Content Determination
Many peptides contain moisture, salts, or counterions that contribute to total weight but aren’t the active peptide. Peptide content specifies the actual peptide percentage by weight.
For example, a product might be 98% pure by HPLC but contain only 80% peptide content by weight due to moisture and salts. For accurate experimental dosing, understanding peptide content is crucial.
High-quality suppliers specify peptide content on CoAs, typically using methods like amino acid analysis or quantitative amino acid determination. When peptide content isn’t specified, researchers may need to assume lower actual peptide amounts and adjust dosing accordingly.
Additional Testing
Depending on the application and purity grade, additional testing might include endotoxin testing (for in vivo applications, as endotoxins can trigger immune responses), sterility testing (for pharmaceutical-grade peptides), residual solvent analysis (ensuring synthesis solvents have been adequately removed), and counter-ion identification (specifying which salts are present).
Evaluating Peptide Suppliers

Not all peptide suppliers are equal. Evaluating suppliers comprehensively helps ensure consistent quality and reliable service.
Quality Management Systems
Reputable suppliers maintain robust quality management systems including documented synthesis and purification protocols, comprehensive testing for every batch, proper storage and handling procedures, stability testing programs, and quality control oversight.
Ask suppliers about their quality systems. ISO 9001 certification (quality management standard), ISO 13485 certification (medical device quality management, relevant for pharmaceutical applications), and GMP facilities for pharmaceutical-grade peptides indicate serious quality commitments.
Technical Expertise and Support
Quality suppliers employ scientists who understand peptide chemistry and can provide technical consultation on peptide selection, advice on handling and storage, troubleshooting support when issues arise, and custom synthesis capabilities for special requirements.
Responsiveness to technical questions before purchase indicates how suppliers will support customers after sale.
Transparency and Documentation
Quality suppliers provide comprehensive documentation including detailed CoAs with all relevant testing data, clear specifications for purity grade and content, proper storage and handling instructions, and safety data sheets (SDS) for hazard information.
Suppliers who hesitate to provide documentation or offer vague responses about testing methods should raise concerns.
Reputation and Reviews
Research supplier reputations through scientific community feedback on forums and social media, published research citing the supplier, customer reviews and testimonials, and length of time in business (longevity often indicates reliability).
While newer suppliers can be excellent, established suppliers with track records provide more confidence.
Customer Service and Reliability
Beyond product quality, reliable suppliers demonstrate prompt communication and order processing, accurate order fulfillment, proper packaging protecting peptides during shipping, reasonable shipping times, and fair policies for handling quality issues.
Pricing Considerations
While price shouldn’t be the sole criterion, understanding pricing helps assess value. Suspiciously low prices may indicate quality compromises, appropriate pricing reflects synthesis complexity and purity level, and extremely high prices don’t necessarily guarantee better quality.
Compare prices across reputable suppliers, but prioritize quality verification over cost alone. The expense of failed experiments due to poor-quality peptides far exceeds the cost difference between quality grades.
Red Flags to Watch For
Certain warning signs should prompt caution or reconsideration:
Lack of Testing DocumentProper Peptide Storage and Handlingation
Suppliers who don’t provide CoAs or provide minimal testing information may not adequately test products. Without documentation, quality claims cannot be verified.
Inconsistent Quality:
Batch-to-batch inconsistency, where different purchases of the same peptide show variable purity or properties, indicates inadequate quality control.
Unavailable Technical Support
Inability or unwillingness to answer technical questions suggests limited expertise or questionable business practices.
Unclear Storage or Handling Information
Quality peptides require specific storage conditions. Suppliers not providing clear guidance may not understand proper peptide handling.
Too-Good-to-Be-True Claims
Claims of pharmaceutical-grade quality at research-grade prices, or promises of impossible specifications, warrant skepticism.
Poor Communication
Unresponsive customer service, vague answers to specific questions, or reluctance to provide information suggests potential problems.
No Established Business Presence
Suppliers without verifiable business addresses, professional websites, or established presence may not be reliable long-term partners.
Understanding Peptide Modifications
Many research applications require modified peptides with altered properties. Quality suppliers offer various modifications and understand their implications.
Common Modifications
Acetylation (N-terminal acetyl group, often improving stability), amidation (C-terminal amide, preventing degradation), biotinylation (attaching biotin for detection or purification), fluorescent labels (for visualization and tracking), PEGylation (attaching polyethylene glycol chains to improve stability and half-life), cyclization (forming disulfide bonds or other linkages creating cyclic structures), and D-amino acid substitutions (replacing L-amino acids with D-forms, typically increasing stability).
Each modification affects synthesis difficulty, purification requirements, and final purity. Reputable suppliers understand these technical aspects and can advise on appropriate modifications for specific applications.
Verification of Modifications
Modified peptides require additional analytical confirmation. CoAs for modified peptides should include MS data confirming the modification (reflected in molecular weight), HPLC showing appropriate retention time changes, and when relevant, functional testing confirming the modification achieves its intended purpose.
Custom Peptide Synthesis
While many common peptides are available as catalog products, specialized research often requires custom synthesis.
When Custom Synthesis Is Needed
Custom synthesis becomes necessary for novel sequences not commercially available, specific modifications or labels, unusual amino acids or non-natural building blocks, specific purity requirements, and large-scale quantities for extensive studies.
Evaluating Custom Synthesis Capabilities
Quality custom synthesis providers offer feasibility assessment before committing to synthesis, clear timelines and pricing, comprehensive analytical data upon delivery, and scale-up capabilities if moving from small to large quantities.
Custom synthesis typically costs more and takes longer than catalog products but provides precisely what research requires.
Proper Peptide Storage and Handling
Even the highest-quality peptides degrade if improperly handled. Protecting peptide integrity from purchase through use is essential.
Storage Conditions
Lyophilized (freeze-dried) peptides typically require storage at -20°C or -80°C to minimize degradation. Protect from moisture by storing in desiccated environments or with desiccant packets. Keep away from light, as many peptides are photosensitive. Original sealed vials generally maintain stability longer than opened containers.
Reconstitution Best Practices
When reconstituting peptides, use appropriate solvents based on peptide properties—hydrophilic peptides often dissolve in water or buffers, while hydrophobic peptides may require organic solvents or surfactants. Add solvent gently, avoiding vigorous mixing that might denature or aggregate peptides. Allow adequate time for dissolution; some peptides dissolve slowly. Confirm complete dissolution before use, as undissolved material indicates potential problems.
Aliquoting for Long-Term Storage
Repeated freeze-thaw cycles degrade many peptides. After reconstitution, aliquoting the solution into single-use portions maintains stability by avoiding repeated freeze-thaw, protecting unused material from degradation, and facilitating convenient use.
Stability Considerations
Peptide stability varies widely depending on sequence, environment, and storage conditions. Sequences containing methionine, cysteine, or tryptophan are particularly prone to oxidation. Peptides with asparagine or glutamine may undergo deamidation. Peptides with aspartic acid followed by proline are susceptible to hydrolysis.
Quality suppliers can provide stability data or guidance on expected stability under various conditions. For critical applications, confirming peptide integrity before use (by HPLC or other methods) ensures quality.
Documentation and Record-Keeping
Maintaining thorough records of peptide purchases and handling supports research integrity and troubleshooting.
Essential Records
Document batch numbers for all peptides used, enabling traceability if issues arise later. Keep CoAs for reference, particularly HPLC and MS data. Record reconstitution details including solvent, concentration, and date. Note storage conditions and any deviations. Track freeze-thaw cycles if applicable. Document any observations about solubility, appearance, or unusual properties.
This documentation helps troubleshoot unexpected results, ensures reproducibility, supports publication data, and enables communication with suppliers if quality concerns arise.
Regulatory Considerations for Research Peptides
Peptides used in research, particularly in vivo or clinical applications, face regulatory considerations.
Research Use Only
Most research-grade peptides are labeled “For Research Use Only” (RUO), meaning they haven’t been approved for human therapeutic use. Using RUO peptides outside proper research contexts violates regulations. Institutional oversight (IACUC for animal research, IRB for human subjects) is required. Proper safety protocols must be followed.
Clinical and GLP Studies
Research advancing toward human applications requires pharmaceutical-grade peptides manufactured under GMP, comprehensive testing including safety and purity analyses, proper regulatory documentation, and sourcing from suppliers with regulatory experience.
Understanding regulatory requirements early prevents costly material changes as research progresses through development stages.
Cost-Benefit Analysis
Balancing quality and cost requires considering the full picture beyond purchase price.
True Cost of Quality
The cheapest peptide isn’t necessarily the most economical choice when accounting for risk of failed experiments requiring repetition, time wasted troubleshooting quality issues, potentially compromised research conclusions, and possible need to repeat entire studies if quality concerns emerge later.
High-quality peptides from reputable suppliers typically cost more upfront but reduce risk and support efficient research progress.
When to Prioritize Quality
Certain situations warrant prioritizing quality even if more expensive: pivotal experiments critical to research conclusions, studies intended for publication in high-impact journals, research leading toward clinical applications or commercialization, work with expensive or limited biological samples, and studies where reproducibility is paramount.
When Lower Grades May Suffice
In some situations, lower purity grades or less expensive suppliers might be appropriate: very preliminary screening studies, large-scale applications where cost is prohibitive, research questions not sensitive to minor impurities, and situations where findings will be confirmed with higher-quality materials before drawing conclusions.
Thoughtful assessment of specific research needs guides appropriate quality level selection.
Building Supplier Relationships
Establishing relationships with reliable suppliers benefits long-term research programs.
Benefits of Consistency
Using consistent, reliable suppliers provides familiarity with their quality standards, streamlined ordering and communication, potential volume discounts for ongoing needs, and trusted partners for technical consultation.
Providing Feedback
Quality suppliers value customer feedback. Reporting both positive experiences and concerns helps suppliers maintain quality, enables addressing issues promptly, and contributes to overall market quality.
Conclusion
Choosing quality research peptides requires unde roper handling practices. While the peptide market offers numerous options at varying quality levels, investing time in supplier evaluation and quality verification pays dividends through reliable data, efficient research progress, and confidence in conclusions.
The key principles include prioritizing appropriate quality levels for specific applications, verifying quality through comprehensive CoA review, evaluating suppliers holistically beyond price alone, handling and storing peptides properly to maintain integrity, and documenting everything to support reproducibility and troubleshooting.
By applying these principles, researchers can navigate the peptide marketplace effectively, selecting materials that support high-quality research and contribute to scientific progress. Quality peptides form the foundation for reliable research, making supplier selection one of the most important decisions in peptide-based experimental work.