The glucagon-like peptide (GLP) family has revolutionized metabolic medicine, offering powerful tools for managing diabetes, obesity, and related conditions. While GLP-1 agonists have become household names with drugs like semaglutide (Ozempic/Wegovy) and liraglutide (Victoza/Saxenda), newer compounds like GLP-3 are expanding our understanding of metabolic regulation. This comprehensive comparison explores how these peptides work, their applications, and what distinguishes GLP-3 from its better-known cousin.
Understanding the GLP Family
Glucagon-like peptides belong to the incretin hormone family, substances released from the gut in response to food intake that enhance insulin secretion. These peptides play crucial roles in regulating blood sugar, appetite, digestion, and metabolism.
The GLP system evolved as a sophisticated mechanism for coordinating energy intake with energy storage and utilization. When you eat, specialized cells in the intestine release GLP hormones that signal various organs about incoming nutrients, coordinating appropriate metabolic responses.
GLP peptides bind to specific receptors on cell surfaces, triggering cascades of cellular events. These receptors exist on pancreatic beta cells (which produce insulin), brain regions controlling appetite and reward, the stomaComparing Mechanisms of Actionch and intestines, liver, heart, and other tissues. This widespread receptor distribution explains GLPs’ diverse effects throughout the body.
GLP-1: The Well-Established Player
GLP-1 (glucagon-like peptide-1) is an incretin hormone produced primarily in intestinal L-cells in response to food intake. It has become the focus of intense pharmaceutical development, resulting in numerous FDA-approved drugs for diabetes and obesity.
GLP-1’s primary mechanisms include stimulating insulin secretion when blood glucose is elevated (glucose-dependent insulin secretion), suppressing glucagon release (glucagon raises blood sugar, so suppressing it helps lower glucose), slowing gastric emptying (keeping food in the stomach longer, reducing post-meal glucose spikes), reducing appetite through effects on brain appetite centers, and potentially preserving pancreatic beta cell function.
Natural GLP-1 has a very short half-life—only minutes—because an enzyme called DPP-4 rapidly breaks it down. This necessitated developing modified GLP-1 agonists resistant to DPP-4 degradation, allowing once-daily or even once-weekly dosing.
FDA-approved GLP-1 agonists include exenatide (Byetta, Bydureon), liraglutide (Victoza, Saxenda), semaglutide (Ozempic, Wegovy, Rybelsus), dulaglutide (Trulicity), and others. These medications have transformed diabetes and obesity treatment, offering significant benefits in glucose control, weight loss, and cardiovascular protection.
Clinical benefits of GLP-1 agonists extend beyond glucose lowering. Studies demonstrate significant weight loss (10-15% or more with higher doses), reduced cardiovascular events including heart attacks and strokes, potential kidney protection in diabetic patients, reduced fatty liver disease severity, and improvements in metabolic markers including blood pressure and lipids.
Introducing GLP-3 RT
GLP-3 represents a newer area of research in the GLP family. While less extensively studied than GLP-1, emerging research suggests GLP-3 may offer unique benefits and mechanisms distinct from GLP-1.
The “RT” designation typically refers to “research” or potentially to specific modifications enhancing the peptide’s properties. Unlike GLP-1, which has extensive clinical data and FDA-approved formulations, GLP-3 remains primarily in research phases, with ongoing investigation into its mechanisms and applications.
GLP-3 appears to interact with related but potentially distinct receptor pathways compared to GLP-1. Early research suggests it may offer complementary metabolic benefits, different tissue distribution and action compared to GLP-1, potentially unique effects on energy metabolism, and possible applications beyond traditional GLP-1 uses.
Understanding GLP-3 requires recognizing that the glucagon-like peptide family is complex, with multiple peptides derived from the same precursor protein through different processing pathways. GLP-3 represents one such variant, with properties that researchers are still elucidating.
Comparing Mechanisms of Action
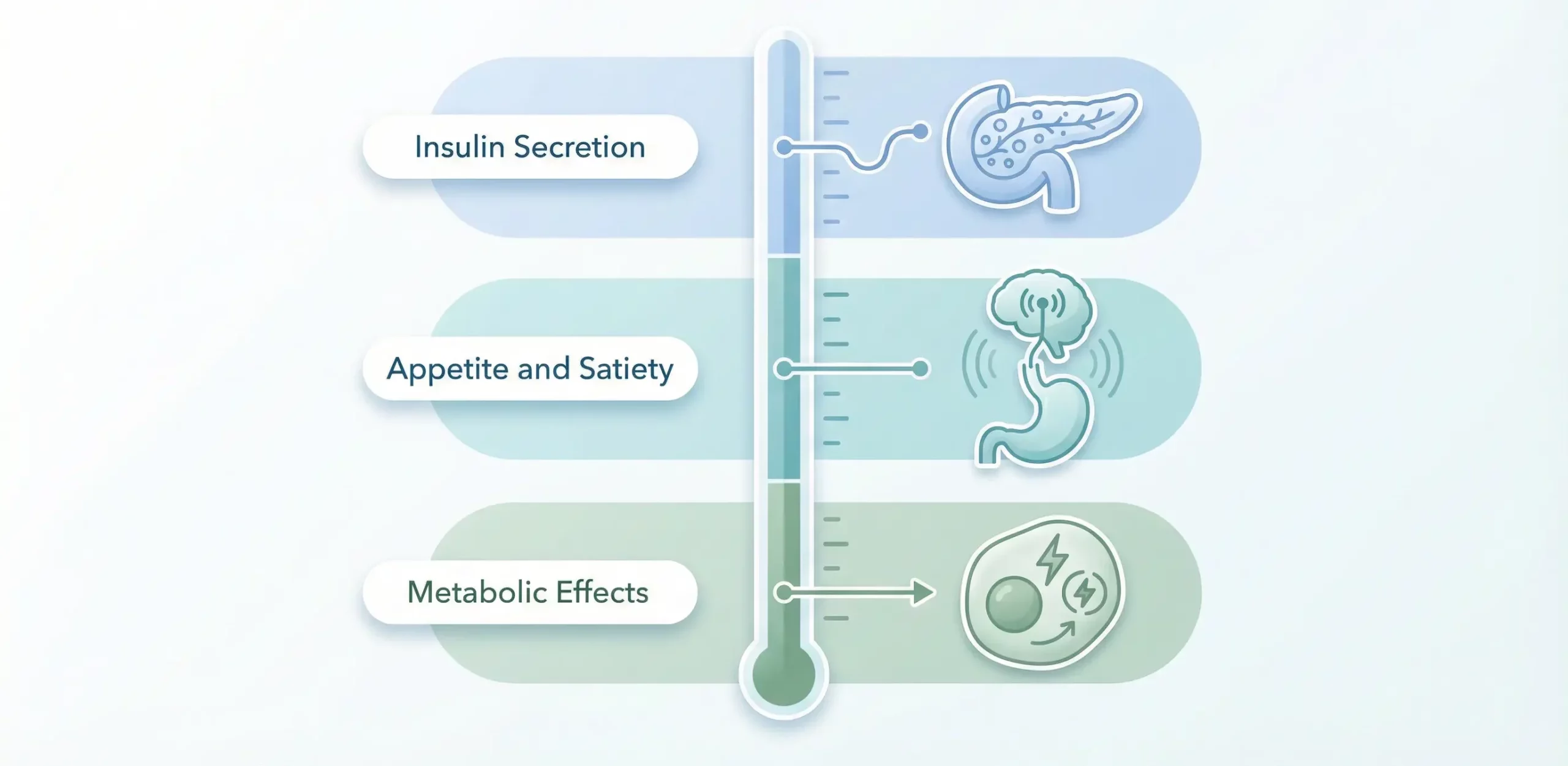
While both GLP-1 and GLP-3 influence metabolic function, their specific mechanisms show important differences:
Insulin Secretion
GLP-1 powerfully stimulates insulin secretion in a glucose-dependent manner, meaning it only enhances insulin release when blood glucose is elevated. This glucose dependency reduces hypoglycemia risk compared to insulin or sulfonylureas. GLP-3’s effects on insulin secretion are under investigation, with early research suggesting it may influence insulin sensitivity through somewhat different pathways.Appetite and Satiety
GLP-1’s appetite-suppressing effects occur primarily through direct action on hypothalamic appetite centers and through slowing gastric emptying. These combined effects powerfully reduce food intake and drive weight loss. GLP-3 may affect appetite through partially distinct mechanisms, potentially offering complementary benefits when combined with other interventions.Metabolic Effects
Both peptides influence whole-body metabolism, but GLP-3 may have unique effects on energy expenditure, fat oxidation, and metabolic flexibility—the ability to switch between burning carbohydrates and fats. These effects could potentially address aspects of metabolic dysfunction not fully corrected by GLP-1 alone.Applications in Diabetes Management
GLP-1 agonists have become cornerstone treatments for type 2 diabetes, recommended by treatment guidelines as preferred options for many patients, particularly those with cardiovascular disease or obesity.
GLP-1’s diabetes benefits include substantial A1C reductions (typically 1-1.5% or more), low hypoglycemia risk due to glucose-dependent mechanism, weight loss rather than weight gain (unlike insulin and many other diabetes medications), cardiovascular protection demonstrated in outcome trials, and potential slowing of disease progression by preserving beta cell function.
GLP-3 research in diabetes is more preliminary. Studies are exploring whether GLP-3 might enhance insulin sensitivity through mechanisms distinct from GLP-1’s primary effects on insulin secretion, offer benefits for patients who don’t respond optimally to GLP-1 agonists, provide complementary effects when used alongside other treatments, or address specific aspects of metabolic dysfunction in diabetes.
Some research suggests GLP-3 may particularly influence hepatic (liver) glucose production and peripheral insulin sensitivity, potentially complementing GLP-1’s effects on insulin secretion and appetite.
Weight Management and Obesity
GLP-1 agonists have revolutionized obesity treatment, offering the first medications producing weight loss comparable to bariatric surgery. Semaglutide at 2.4mg weekly (Wegovy) produces average weight loss of 15-17% in clinical trials, with many patients losing more than 20% of body weight.
GLP-1’s weight loss mechanisms include powerful appetite suppression through central nervous system effects, delayed gastric emptying reducing meal size and frequency, alterations in food preferences (reduced preference for high-fat, high-sugar foods), increased satiety and reduced hunger between meals, and possible modest increases in energy expenditure.
GLP-3’s role in weight management remains under investigation. Research questions include whether GLP-3 affects body composition differently than GLP-1, potentially preserving more muscle mass during weight loss, if GLP-3 enhances fat oxidation and metabolic rate through distinct pathways, whether combining GLP-3 with GLP-1 approaches offers advantages over either alone, and how GLP-3 might benefit metabolic flexibility and long-term weight maintenance.
Early research suggests GLP-3 may have particular effects on fat metabolism and energy expenditure that could complement the appetite-suppressing effects of GLP-1, potentially offering a more comprehensive approach to obesity treatment.
Cardiovascular Benefits
One of the most important discoveries about GLP-1 agonists was their cardiovascular protective effects. Large outcome trials demonstrated that several GLP-1 agonists significantly reduce major adverse cardiovascular events (heart attack, stroke, cardiovascular death) in patients with type 2 diabetes.
These cardiovascular benefits occur through multiple mechanisms: improvements in traditional risk factors (glucose, weight, blood pressure, lipids), direct effects on the cardiovascular system including improved endothelial function, reduced inflammation throughout the cardiovascular system, beneficial effects on heart muscle and cardiac function, and potential anti-atherosclerotic effects.
GLP-3’s cardiovascular effects are less well-studied. Research is exploring whether GLP-3 might offer cardiovascular protection through mechanisms distinct from GLP-1, complement GLP-1’s cardiovascular benefits, have unique effects on heart metabolism or function, and provide benefits in cardiovascular conditions where metabolic dysfunction plays a role.
Given the importance of cardiovascular disease as a leading cause of death globally, any compound offering cardiovascular protection merits serious investigation.
Side Effects and Tolerability
GLP-1 agonists’ side effects are well-characterized from extensive clinical use. The most common are gastrointestinal: nausea (experienced by 20-40% of patients, usually diminishing over time), vomiting, diarrhea, constipation, abdominal discomfort, and reduced appetite (which contributes to therapeutic effects but can be excessive for some patients).
Most GI side effects are dose-dependent and improve with gradual dose titration. Starting at low doses and slowly increasing over weeks to months significantly improves tolerability.
Less common but important GLP-1 agonist side effects include increased heart rate in some patients, potential increased risk of gallbladder disease with rapid weight loss, and theoretical concerns about pancreatitis and thyroid C-cell tumors (though these risks remain controversial and may not be clinically significant).
GLP-3’s side effect profile is less well-defined due to limited clinical use. Research aims to determine whether GLP-3 causes similar GI side effects as GLP-1, if tolerability differs between the peptides, what optimal dosing strategies minimize side effects, and if GLP-3 might suit patients who don’t tolerate GLP-1 agonists well.
Understanding side effect profiles helps guide patient selection and management strategies, ensuring that benefits outweigh risks for individual patients.
Dosing and Administration
GLP-1 agonists use various dosing strategies. Immediate-release formulations like exenatide (Byetta) require twice-daily injection. Extended-release formulations like exenatide XR (Bydureon), semaglutide (Ozempic, Wegovy), and dulaglutide (Trulicity) use once-weekly injection. Oral semaglutide (Rybelsus) offers the first oral GLP-1 option, taken daily on an empty stomach.
Dose titration is crucial for GLP-1 agonists. Starting at low doses and gradually increasing over weeks to months improves tolerability by allowing GI adaptation. Most GLP-1 agonists have defined titration schedules based on clinical trial data.
GLP-3 dosing protocols are still being established in research contexts. Studies are exploring optimal dose ranges for different applications, whether dosing frequency differs from GLP-1 agonists, if GLP-3 can be formulated for convenient administration, and how to optimize combination approaches involving GLP-3.
Potential Combination Approaches
An intriguing research question is whether combining GLP-1 and GLP-3, or using multi-agonist approaches, might offer advantages over single peptides alone.
The pharmaceutical industry has already developed dual agonists targeting both GLP-1 and GIP (glucose-dependent insulinotropic polypeptide) receptors. Tirzepatide (Mounjaro, Zepbound), a GLP-1/GIP dual agonist, produces greater weight loss and glucose reduction than GLP-1 agonists alone, validating the multi-agonist concept.
Triple agonists targeting GLP-1, GIP, and glucagon receptors are in development, with early data suggesting even greater metabolic benefits.
GLP-3 might fit into combination strategies by providing complementary metabolic effects not fully achieved by GLP-1 alone, potentially enhancing specific aspects like fat oxidation or metabolic flexibility, possibly allowing lower doses of each peptide with fewer side effects, or addressing metabolic resistance that can develop with single-peptide approaches.
Research into optimal combination strategies continues, with the goal of maximizing metabolic benefits while minimizing side effects.
Research Status and Future Directions
GLP-1 agonists are FDA-approved, widely prescribed medications with extensive safety and efficacy data. Ongoing research explores new formulations (oral, transdermal), longer-acting versions, combinations with other drug classes, expanded indications (heart failure, kidney disease, neurodegenerative conditions), and personalized medicine approaches identifying which patients benefit most.
GLP-3 research is earlier-stage, primarily involving preclinical and early clinical studies. Research priorities include fully characterizing GLP-3’s mechanisms and receptor interactions, establishing optimal dosing and administration protocols, conducting larger-scale clinical trials for various metabolic conditions, exploring combination approaches with GLP-1 and other interventions, and identifying patient populations most likely to benefit.
As GLP-3 research advances, we’ll better understand where it fits in the metabolic medicine toolbox and whether it offers meaningful advantages over or complements existing GLP-1 therapies.
Practical Considerations
For patients and healthcare providers, GLP-1 agonists represent proven, accessible options for diabetes and obesity management. With multiple approved medications and growing insurance coverage, they’re readily available for appropriate patients.
GLP-3 remains primarily in research contexts. Individuals interested in GLP-3 should understand its experimental status and limited clinical data, work with knowledgeable providers familiar with peptide research, consider participation in clinical trials if available, and maintain realistic expectations about potential benefits and risks.
For researchers and enthusiasts exploring peptides, understanding both GLP-1 and GLP-3 provides context for metabolic regulation and the evolving landscape of metabolic therapeutics.
Conclusion
GLP-1 and GLP-3 represent different points in the continuum of metabolic peptide development. GLP-1 agonists have proven revolutionary for diabetes and obesity treatment, with extensive evidence supporting their efficacy and safety. GLP-3 represents an intriguing research avenue that may eventually expand our therapeutic options for metabolic disease.
Understanding the mechanisms, applications, and differences between these peptides helps contextualize current treatments and future possibilities. Whether GLP-3 ultimately joins GLP-1 as a widely-used therapeutic agent remains to be determined by ongoing research. Meanwhile, GLP-1 agonists continue transforming metabolic medicine, offering powerful tools for managing conditions that affect hundreds of millions globally.
As research progresses, the GLP family may expand further, potentially offering increasingly sophisticated and personalized approaches to metabolic health optimization.