Mitochondria, often called the powerhouses of our cells, play a crucial role in energy production, cellular signaling, and overall health. When mitochondrial function declines, it contributes to aging, chronic diseases, and reduced cellular performance. SS-31 peptide, also known as elamipretide or Bendavia, has emerged as a promising compound for supporting mitochondrial health and function. This comprehensive guide explores the science of mitochondria, how SS-31 works, and what research reveals about its potential applications.
Understanding Mitochondrial Function and Dysfunction
Mitochondria are specialized structures within cells responsible for producing adenosine triphosphate (ATP), the energy currency that powers virtually all cellular processes. A single cell may contain hundreds to thousands of mitochondria, with energy-demanding tissues like the heart, brain, and muscles containing the highest concentrations.
Beyond energy production, mitochondria regulate cellular metabolism, calcium signaling, apoptosis (programmed cell death), and production of reactive oxygen species (ROS). When mitochondrial function becomes impaired, these critical processes suffer, leading to cellular dysfunction and disease.
Mitochondrial dysfunction manifests in various ways. Energy production declines, leaving cells unable to meet metabolic demands. Oxidative stress increases as damaged mitochondria produce excess ROS while antioxidant defenses weaken. Cellular signaling becomes disrupted, affecting everything from gene expression to immune responses.
Multiple factors contribute to mitochondrial dysfunction. Aging naturally degrades mitochondrial function through accumulated damage to mitochondrial DNA, proteins, and lipids. Environmental toxins, poor nutrition, chronic stress, and sedentary lifestyles all accelerate mitochondrial decline. Genetic mutations affecting mitochondrial function cause primary mitochondrial diseases, while acquired mitochondrial dysfunction contributes to common conditions including heart disease, neurodegenerative diseases, diabetes, and metabolic syndrome.
The Science Behind SS-31 Peptide
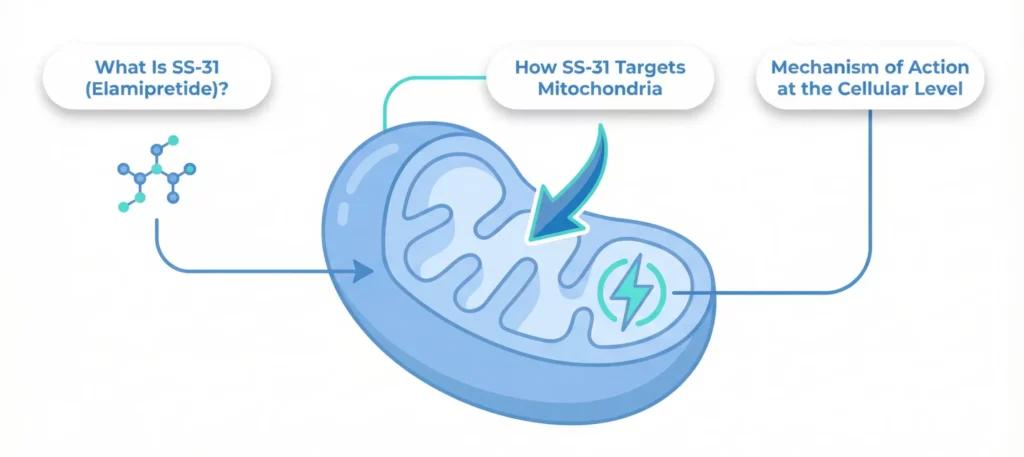
SS-31 peptide represents a novel approach to supporting mitochondrial health by targeting the inner mitochondrial membrane where energy production occurs. This small, water-soluble tetrapeptide (consisting of four amino acids: D-Arg-Dmt-Lys-Phe-NH2) possesses unique properties that allow it to selectively accumulate in mitochondria.
The peptide’s mechanism centers on its interaction with cardiolipin, a phospholipid found almost exclusively in the inner mitochondrial membrane. Cardiolipin plays essential roles in mitochondrial structure and function, organizing protein complexes involved in energy production, stabilizing mitochondrial membranes, and regulating various mitochondrial processes.
In dysfunctional mitochondria, cardiolipin becomes oxidized and damaged, leading to disrupted energy production, increased ROS generation, and structural instability. SS-31 binds to cardiolipin, protecting it from oxidative damage and stabilizing the inner mitochondrial membrane. This protection helps maintain the structure and function of electron transport chain complexes, improving energy production efficiency while reducing damaging ROS generation.
Research indicates SS-31 does not act as a traditional antioxidant that scavenges free radicals. Instead, it prevents ROS generation at the source by optimizing mitochondrial function. This distinction is important because traditional antioxidants have shown limited effectiveness in treating conditions involving mitochondrial dysfunction, possibly because they don’t address the root cause of excessive ROS production.
Research on SS-31 in Cardiovascular Health
The heart, with its enormous energy demands, depends critically on optimal mitochondrial function. Consequently, much SS-31 research has focused on cardiovascular applications.
Studies in heart failure models have demonstrated that SS-31 can improve cardiac function, reduce damage from ischemia-reperfusion injury (the damage that occurs when blood flow returns after a period of reduced oxygen), and protect heart cells from death. In animal models, SS-31 treatment improved left ventricular function, reduced infarct size following heart attacks, and enhanced exercise capacity.
Clinical trials in humans have explored SS-31 for several cardiovascular conditions. In patients with heart failure with preserved ejection fraction (HFpEF), a condition where the heart pumps normally but doesn’t fill properly, SS-31 showed promise in improving diastolic function and exercise capacity in early-phase trials. Research in patients undergoing cardiac surgery examined whether SS-31 could reduce organ injury associated with the procedure.
The peptide’s cardiovascular benefits appear to stem from multiple mechanisms: improving mitochondrial energy production, reducing oxidative stress in cardiac tissue, protecting against ischemia-reperfusion injury, preserving cardiac cell viability, and maintaining healthy endothelial function in blood vessels.
Neurological Applications and Brain Health
The brain’s high energy demands and limited regenerative capacity make it particularly vulnerable to mitochondrial dysfunction. Neurodegenerative diseases including Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis (ALS) all involve mitochondrial impairment as a key pathological feature.
Research suggests SS-31 may offer neuroprotective benefits through several pathways. By improving neuronal energy production, the peptide helps maintain the high metabolic demands of brain cells. Reducing oxidative stress in the brain, where neurons are particularly vulnerable to ROS damage, protects neural tissue. The peptide may also support synaptic function—the connections between neurons critical for cognition and memory—and protect against neuronal death in various disease models.
Animal studies have shown promising results. In models of Alzheimer’s disease, SS-31 treatment reduced amyloid plaque formation, improved cognitive function, and protected neurons from degeneration. Parkinson’s disease models showed that SS-31 protected dopamine-producing neurons that are lost in this condition, improved motor function, and reduced neuroinflammation.
Traumatic brain injury research has also explored SS-31, finding it may reduce secondary injury following trauma, protect neural tissue, improve recovery outcomes, and reduce long-term neurological deficits.
Metabolic Health and Diabetes Research
Mitochondrial dysfunction plays a central role in metabolic diseases, particularly type 2 diabetes and metabolic syndrome. Impaired mitochondrial function in muscle, fat, and liver tissue contributes to insulin resistance, reduced fat oxidation, and metabolic inflexibility—the inability to efficiently switch between burning carbohydrates and fats.
SS-31 research in metabolic health has examined multiple aspects. Studies show the peptide may improve insulin sensitivity by enhancing mitochondrial function in insulin-responsive tissues, support healthy glucose metabolism by optimizing cellular energy production, improve fat oxidation helping address obesity and metabolic syndrome, and reduce inflammation associated with metabolic dysfunction.
In diabetic animal models, SS-31 treatment improved glucose control, enhanced insulin sensitivity, reduced fatty liver disease progression, improved muscle mitochondrial function, and supKidney Disease and Organ Protectionported healthier body composition.
Skeletal muscle represents a major site of glucose disposal and energy expenditure. By improving mitochondrial function in muscle tissue, SS-31 may help restore metabolic flexibility and improve overall metabolic health. This has implications not only for diabetes but also for obesity, metabolic syndrome, and age-related metabolic decline.
Aging and Longevity Research
The mitochondrial theory of aging proposes that accumulated mitochondrial damage drives the aging process. As mitochondrial function declines with age, cells lose energy production capacity, oxidative stress increases, and age-related diseases develop.
SS-31 research in aging models has yielded intriguing results. Studies in aged animals show that SS-31 treatment can improve physical performance and endurance, enhance cognitive function, reduce age-related cellular damage, improve tissue health across multiple organ systems, and potentially extend healthspan—the period of healthy life.
Importantly, research suggests SS-31 doesn’t simply extend lifespan but rather improves the quality of life during aging, maintaining function and reducing disease burden. This distinction matters enormously for translating research into meaningful human benefits.
Age-related muscle loss (sarcopenia) represents another area where SS-31 shows promise. By improving mitochondrial function in muscle tissue, the peptide may help preserve muscle mass and strength during aging, supporting independence and quality of life.
Kidney Disease and Organ Protection
Kidneys contain abundant mitochondria to power the energy-intensive processes of blood filtration and electrolyte regulation. Mitochondrial dysfunction contributes to various kidney diseases, and SS-31 has shown protective effects in renal research.
Studies demonstrate SS-31 may protect kidneys from acute injury caused by reduced blood flow, toxins, or contrast agents used in medical imaging. The peptide appears to reduce kidney cell death, preserve kidney function, minimize inflammation, and support recovery from acute kidney injury.
In chronic kidney disease models, SS-31 treatment slowed disease progression, preserved kidney structure, reduced fibrosis (scarring), and maintained better kidney function compared to untreated controls.
Exercise Performance and Muscle Function
Athletes and fitness enthusiasts have shown interest in SS-31 for its potential to enhance mitochondrial function in muscle tissue. While much research remains preliminary, studies suggest several possible benefits.
Animal studies show SS-31 may improve exercise endurance by enhancing mitochondrial efficiency in muscle cells, support faster recovery by reducing exercise-induced oxidative stress, maintain muscle function by protecting mitochondria from damage, and potentially improve the adaptive response to exercise training.
However, it’s crucial to note that most human research has focused on disease conditions rather than performance enhancement in healthy individuals. The ethical and regulatory considerations around using peptides for performance enhancement remain complex and evolving.
Safety Profile and Research Considerations
Clinical trials examining SS-31 have generally reported favorable safety profiles. Most adverse events in trials were mild and not clearly related to the peptide. However, as with any compound under investigation, long-term safety data remains limited, and ongoing research continues evaluating safety across different populations and conditions.
The peptide’s selective targeting of mitochondria, rather than broadly affecting cellular processes, may contribute to its apparently favorable safety profile. Because SS-31 doesn’t accumulate in tissues and is relatively quickly eliminated from the body, it presents a lower risk of long-term accumulation and toxicity.
Current research limitations include the need for larger, longer-term human clinical trials to fully establish efficacy and safety, better understanding of optimal dosing regimens for different conditions, identification of which patient populations benefit most from treatment, and clarification of the peptide’s effects when used long-term.
Current Research Status and Future Directions
SS-31 research continues advancing, with multiple clinical trials underway or completed examining various applications. Some trials have shown promising results, while others have not met primary endpoints, highlighting the complexity of translating preclinical findings to human patients.
The pharmaceutical company developing SS-31 (under the name elamipretide) for clinical use has pursued FDA approval for specific indications, particularly primary mitochondrial diseases. The regulatory pathway for peptide therapeutics presents unique challenges, and approval timelines remain uncertain.
Future research directions include identifying biomarkers that predict which patients will respond to SS-31 treatment, exploring combination therapies that pair SS-31 with other interventions, developing next-generation mitochondrial-targeted peptides with improved properties, and expanding research into additional disease applications where mitochondrial dysfunction plays a role.
Practical Implications and Considerations
For individuals interested in mitochondrial health, SS-31 represents one tool among many for supporting mitochondrial function. However, it’s important to maintain perspective. Lifestyle factors profoundly influence mitochondrial health, and no peptide can substitute for foundational health practices.
Exercise, particularly high-intensity interval training and resistance training, represents perhaps the most powerful stimulus for improving mitochondrial function. Regular physical activity increases mitochondrial number and efficiency, improves oxidative capacity, and enhances metabolic flexibility.
Nutrition significantly affects mitochondrial health. Adequate intake of B vitamins, coenzyme Q10, magnesium, and other micronutrients supports mitochondrial function. Avoiding excessive calorie intake and processed foods reduces metabolic stress on mitochondria.
Sleep quality affects mitochondrial health, with poor sleep associated with mitochondrial dysfunction. Managing stress, avoiding environmental toxins, and maintaining healthy body composition all contribute to optimal mitochondrial function.